The significance of galectin-3 as a new basal cell marker in prostate cancer
- PMID: 23907467
- PMCID: PMC3763439
- DOI: 10.1038/cddis.2013.277
The significance of galectin-3 as a new basal cell marker in prostate cancer
Abstract
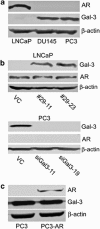
Prostate cancer may originate from distinct cell types, resulting in the heterogeneity of this disease. Galectin-3 (Gal-3) and androgen receptor (AR) have been reported to play important roles in the progression of prostate cancer, and their heterogeneous expressions might be associated with different cancer subtypes. Our study found that in various prostate cancer cell lines Gal-3 expression was always opposite to AR expression and other luminal cell markers but consistent with basal cell markers including glutathione S-transferase-π and Bcl-2. This expression pattern was confirmed in human prostate cancer tissues. Our results also showed that prostate cancer cells positive with basal cell markers were more aggressive. Downregulation of Gal-3 expression resulted in increased apoptotic potential and decreased metastasis potential of prostate cancer cells. Our findings demonstrate for the first time that Gal-3 may serve as a new marker for basal characteristics of prostate cancer epithelium. This study helps us to better understand the heterogeneity of prostate cancer. The clinical significance of this study lies in the application of Gal-3 to distinguish prostate cancer subtypes and improve treatment efficacy with designed personalized therapy.
Figures




References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
-
- Long RM, Morrissey C, Fitzpatrick JM, Watson RW. Prostate epithelial cell differentiation and its relevance to the understanding of prostate cancer therapies. Clin Sci (Lond) 2005;108:1–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

