Lipid-insertion enables targeting functionalization of erythrocyte membrane-cloaked nanoparticles
- PMID: 23907698
- PMCID: PMC3831007
- DOI: 10.1039/c3nr03064d
Lipid-insertion enables targeting functionalization of erythrocyte membrane-cloaked nanoparticles
Abstract
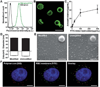
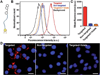
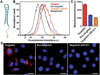
RBC membrane-cloaked polymeric nanoparticles represent an emerging nanocarrier platform with extended circulation in vivo. A lipid-insertion method is employed to functionalize these nanoparticles without the need for direct chemical conjugation. Insertion of both folate and the nucleolin-targeting aptamer AS1411 shows receptor-specific targeting against model cancer cell lines.
Figures

References
-
- Li SD, Huang L. Mol. Pharmaceutics. 2008;5:496–504. - PubMed
-
- Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. J. Controlled Release. 2000;65:271–284. - PubMed
-
- Iyer AK, Khaled G, Fang J, Maeda H. Drug Discovery Today. 2006;11:812–818. - PubMed
-
- Emerich DF, Thanos CG. J. Drug Targeting. 2007;15:163–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

