Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naïve genotype 1 hepatitis C: the randomized PILLAR study
- PMID: 23907700
- PMCID: PMC4112500
- DOI: 10.1002/hep.26641
Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naïve genotype 1 hepatitis C: the randomized PILLAR study
Abstract
The phase IIb, double-blind, placebo-controlled PILLAR trial investigated the efficacy and safety of two different simeprevir (SMV) doses administered once-daily (QD) with pegylated interferon (Peg-IFN)-α-2a and ribavirin (RBV) in treatment-naïve patients with HCV genotype 1 infection. Patients were randomized to one of five treatments: SMV (75 or 150 mg QD) for 12 or 24 weeks or placebo, plus Peg-IFN and RBV. Patients in the SMV arms stopped all treatment at week 24 if response-guided therapy (RGT) criteria were met; patients not meeting RGT continued with Peg-IFN and RBV until week 48, as did patients in the placebo control group. Sustained virologic response (SVR) rates measured 24 weeks after the planned end of treatment (SVR24) were 74.7%-86.1% in the SMV groups versus 64.9% in the control group (P < 0.05 for all comparisons [SMV versus placebo], except SMV 75 mg for 24 weeks). Rapid virologic response (HCV RNA <25 IU/mL undetectable at week 4) was achieved by 68.0%-75.6% of SMV-treated and 5.2% of placebo control patients. According to RGT criteria, 79.2%-86.1% of SMV-treated patients completed treatment by week 24; 85.2%-95.6% of these subsequently achieved SVR24. The adverse event profile was generally similar across the SMV and placebo control groups, with the exception of mild reversible hyperbilirubinemia, without serum aminotransferase abnormalities, associated with higher doses of SMV.
Conclusion: SMV QD in combination with Peg-IFN and RBV significantly improves SVR rates, compared with Peg-IFN and RBV alone, and allows the majority of patients to shorten their therapy duration to 24 weeks.
Trial registration: ClinicalTrials.gov NCT00882908.
© 2013 by the American Association for the Study of Liver Diseases.
Figures

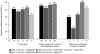

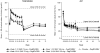

References
-
- Asselah T, Marcellin P. Direct acting antivirals for the treatment of chronic hepatitis C: one pill a day for tomorrow. Liver Int. 2012;32(Suppl 1):88–102. - PubMed
-
- Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–2416. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
