Reversing pathologically increased EEG power by acoustic coordinated reset neuromodulation
- PMID: 23907785
- PMCID: PMC4216412
- DOI: 10.1002/hbm.22314
Reversing pathologically increased EEG power by acoustic coordinated reset neuromodulation
Abstract
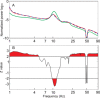
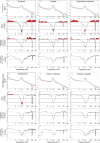
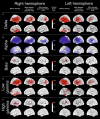
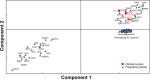
Acoustic Coordinated Reset (CR) neuromodulation is a patterned stimulation with tones adjusted to the patient's dominant tinnitus frequency, which aims at desynchronizing pathological neuronal synchronization. In a recent proof-of-concept study, CR therapy, delivered 4-6 h/day more than 12 weeks, induced a significant clinical improvement along with a significant long-lasting decrease of pathological oscillatory power in the low frequency as well as γ band and an increase of the α power in a network of tinnitus-related brain areas. As yet, it remains unclear whether CR shifts the brain activity toward physiological levels or whether it induces clinically beneficial, but nonetheless abnormal electroencephalographic (EEG) patterns, for example excessively decreased δ and/or γ. Here, we compared the patients' spontaneous EEG data at baseline as well as after 12 weeks of CR therapy with the spontaneous EEG of healthy controls by means of Brain Electrical Source Analysis source montage and standardized low-resolution brain electromagnetic tomography techniques. The relationship between changes in EEG power and clinical scores was investigated using a partial least squares approach. In this way, we show that acoustic CR neuromodulation leads to a normalization of the oscillatory power in the tinnitus-related network of brain areas, most prominently in temporal regions. A positive association was found between the changes in tinnitus severity and the normalization of δ and γ power in the temporal, parietal, and cingulate cortical regions. Our findings demonstrate a widespread CR-induced normalization of EEG power, significantly associated with a reduction of tinnitus severity.
Keywords: desynchronization; electroencephalography; non-invasive neuromodulation; phantom perception; tinnitus treatment.
Copyright © 2013 Wiley Periodicals, Inc.
Conflict of interest statement
I. Adamchic and T. Toth have no conflict of interest in relation to this study. C. Hauptmann and P. A. Tass have a contractual relationship with ANM Adaptive Neuromodulation GmbH. P. A. Tass holds shares of ANM Adaptive Neuromodulation GmbH.
Figures

References
-
- Adamchic I, Langguth B, Hauptmann C, Tass PA (2012a): Psychometric evaluation of Visual Analog Scale for the assessment of chronic tinnitus. Am J Audiol 21:215--225. - PubMed
-
- Benjamini Y, Hochberg Y (1995): Controlling the False Discovery Rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B 57:289–300.
-
- Benoit O, Daurat A, Prado J (2000): Slow (0.7–2 Hz) and fast (2–4 Hz) delta components are differently correlated to theta, alpha and beta frequency bands during NREM sleep. Clin Neurophysiol 111:2103–2106. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

