Miniports versus standard ports for laparoscopic cholecystectomy
- PMID: 23908012
- PMCID: PMC11747961
- DOI: 10.1002/14651858.CD006804.pub3
Miniports versus standard ports for laparoscopic cholecystectomy
Abstract
Background: In conventional (standard) port laparoscopic cholecystectomy, four abdominal ports (two of 10 mm diameter and two of 5 mm diameter) are used. Recently, use of smaller ports, miniports, have been reported.
Objectives: To assess the benefits and harms of miniport (defined as ports smaller than the standard ports) laparoscopic cholecystectomy versus standard port laparoscopic cholecystectomy.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded until February 2013 to identify randomised clinical trials of relevance to this review.
Selection criteria: Only randomised clinical trials (irrespective of language, blinding, or publication status) comparing miniport versus standard port laparoscopic cholecystectomy were considered for the review.
Data collection and analysis: Two review authors collected the data independently. We analysed the data with both fixed-effect and random-effects models using RevMan analysis. For each outcome we calculated the risk ratio (RR), mean difference (MD), or standardised mean difference (SMD) with 95% confidence intervals (CI).
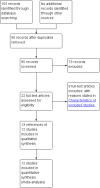
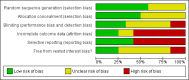
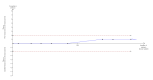
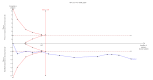
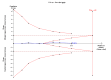
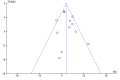
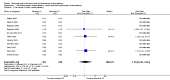
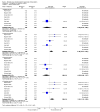
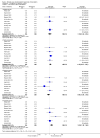
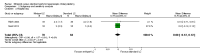
Main results: We included 12 trials with 734 patients randomised to miniport laparoscopic cholecystectomy (380 patients) versus standard laparoscopic cholecystectomy (351 patients). Only one trial which included 70 patients was of low risk of bias. Miniport laparoscopic cholecystectomy could be completed successfully in more than 80% of patients in most trials. The remaining patients were mostly converted to standard port laparoscopic cholecystectomy but some were also converted to open cholecystectomy. These patients were included for the outcome conversion to open cholecystectomy but excluded from other outcomes. Accordingly, the results of the other outcomes are on 343 patients in the miniport laparoscopic cholecystectomy group and 351 patients in the standard port laparoscopic cholecystectomy group, and therefore the results have to be interpreted with extreme caution.There was no mortality in the seven trials that reported mortality (0/194 patients in miniport laparoscopic cholecystectomy versus 0/203 patients in standard port laparoscopic cholecystectomy). There were no significant differences between miniport laparoscopic cholecystectomy and standard laparoscopic cholecystectomy in the proportion of patients who developed serious adverse events (eight trials; 460 patients; RR 0.33; 95% CI 0.04 to 3.08) (miniport laparoscopic cholecystectomy: 1/226 (adjusted proportion 0.4%) versus standard laparoscopic cholecystectomy: 3/234 (1.3%); quality of life at 10 days after surgery (one trial; 70 patients; SMD -0.20; 95% CI -0.68 to 0.27); or in whom the laparoscopic operation had to be converted to open cholecystectomy (11 trials; 670 patients; RR 1.23; 95% CI 0.44 to 3.45) (miniport laparoscopic cholecystectomy: 8/351 (adjusted proportion 2.3%) versus standard laparoscopic cholecystectomy 6/319 (1.9%)). Miniport laparoscopic cholecystectomy took five minutes longer to complete than standard laparoscopic cholecystectomy (12 trials; 695 patients; MD 4.91 minutes; 95% CI 2.38 to 7.44). There were no significant differences between miniport laparoscopic cholecystectomy and standard laparoscopic cholecystectomy in the length of hospital stay (six trials; 351 patients; MD -0.00 days; 95% CI -0.12 to 0.11); the time taken to return to activity (one trial; 52 patients; MD 0.00 days; 95% CI -0.31 to 0.31); or in the time taken for the patient to return to work (two trials; 187 patients; MD 0.28 days; 95% CI -0.44 to 0.99) between the groups. There was no significant difference in the cosmesis scores at six months to 12 months after surgery between the two groups (two trials; 152 patients; SMD 0.13; 95% CI -0.19 to 0.46).
Authors' conclusions: Miniport laparoscopic cholecystectomy can be completed successfully in more than 80% of patients. There appears to be no advantage of miniport laparoscopic cholecystectomy in terms of decreasing mortality, morbidity, hospital stay, return to activity, return to work, or improving cosmesis. On the other hand, there is a modest increase in operating time after miniport laparoscopic cholecystectomy compared with standard port laparoscopic cholecystectomy and the safety of miniport laparoscopic cholecystectomy is yet to be established. Miniport laparoscopic cholecystectomy cannot be recommended routinely outside well-designed randomised clinical trials. Further trials of low risks of bias and low risks of random errors are necessary.
Conflict of interest statement
None known.
Figures







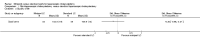








Update of
-
Miniport versus standard ports for laparoscopic cholecystectomy.Cochrane Database Syst Rev. 2010 Mar 17;(3):CD006804. doi: 10.1002/14651858.CD006804.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2013 Aug 01;(8):CD006804. doi: 10.1002/14651858.CD006804.pub3. PMID: 20238350 Updated.
References
References to studies included in this review
Abbas 2009 {published data only}
-
- Abbas MH, Hamade A, Nadeem R, Ammori B. An "all 5‐mm ports" versus conventional ports approach to laparoscopic cholecystectomy and Nissen fundoplication: a randomized clinical trial. Surgical Laparoscopy, Endoscopy and Percutaneous Techniques 2009;19(6):442‐8. - PubMed
Alponat 2002 {published data only (unpublished sought but not used)}
-
- Alponat A, Cubukcu A, Gonullu N, Canturk Z, Ozbay O. Is minisite cholecystectomy less traumatic? Prospective randomized study comparing minisite and conventional laparoscopic cholecystectomies. World Journal of Surgery 2002;26(12):1437‐40. [PUBMED: 12297935] - PubMed
Bisgaard 2000 {published and unpublished data}
-
- Bisgaard T, Klarskov B, Trap R, Kehlet H, Rosenberg J. Pain after microlaparoscopic cholecystectomy. A randomized double‐blind controlled study. Surgical Endoscopy 2000;14(4):340‐4. - PubMed
Bisgaard 2002 {published and unpublished data}
-
- Bisgaard T, Klarskov B, Trap R, Kehlet H, Rosenberg J. Microlaparoscopic vs conventional laparoscopic cholecystectomy ‐ A prospective randomized double‐blind trial. Surgical Endoscopy 2002;16(3):458‐64. - PubMed
de Carvalho 2013 {published data only}
-
- Carvalho LF, Fierens K, Kint M. Mini‐laparoscopic versus conventional laparoscopic cholecystectomy: a randomized controlled trial. Journal of Laparoendoscopic and Advanced Surgical Techniques. Part A 2013;23(2):109‐16. - PubMed
Hsieh 2003 {published data only}
-
- Hsieh CH. Early minilaparoscopic cholecystectomy in patients with acute cholecystitis. American Journal of Surgery 2003;185(4):344‐8. - PubMed
Huang 2003 {published and unpublished data}
-
- Huang MT, Wang W, Wei PL, Chen RJ, Lee WJ. Minilaparoscopic and laparoscopic cholecystectomy: a comparative study. Archives of Surgery 2003;138(9):1017‐23. - PubMed
Novitsky 2005 {published and unpublished data}
-
- Novitsky YW, Czerniach DR, Perugini RA, Yood SM, Kercher KW, Gallagher KA, et al. Prospective randomized trial of mini‐port vs conventional laparoscopic cholecystectomy. Gastroenterology 2002;123(1 Suppl):24.
-
- Novitsky YW, Kercher KW, Czerniach DR, Kaban GK, Khera S, Gallagher DKA, et al. Advantages of mini‐laparoscopic vs conventional laparoscopic cholecystectomy: Results of a prospective randomized trial. Archives of Surgery 2005;140(12):1178‐83. - PubMed
Saad 2013 {published data only}
-
- Saad S, Strassel V, Sauerland S. Randomized clinical trial of single‐port, minilaparoscopic and conventional laparoscopic cholecystectomy. British Journal of Surgery 2013;100(3):339‐49. - PubMed
Sarli 2003 {published data only}
-
- Sarli L, Iusco D, Gobbi S, Porrini C, Ferro M, Roncoroni L. Randomized clinical trial of laparoscopic cholecystectomy performed with mini‐instruments. British Journal of Surgery 2003;90(11):1345‐8. - PubMed
Schmidt 2002 {published data only}
-
- Schmidt J, Sparenberg C, Fraunhofer S, Zirngibl H. Sympathetic nervous system activity during laparoscopic and needlescopic cholecystectomy ‐ A prospective randomized study. Surgical Endoscopy 2002;16(3):476‐80. - PubMed
Schwenk 2000 {published data only}
-
- Schwenk W, Neudecker J, Mall J, Bohm B, Muller JM. Prospective randomized blinded trial of pulmonary function, pain, and cosmetic results after laparoscopic vs. microlaparoscopic cholecystectomy. Surgical Endoscopy 2000;14(4):345‐8. - PubMed
References to studies excluded from this review
Ainslie 2003 {published data only (unpublished sought but not used)}
-
- Ainslie WG, Catton JA, Davides D, Dexter S, Gibson J, Larvin M, et al. Micropuncture cholecystectomy vs conventional laparoscopic cholecystectomy: A randomized controlled trial. Surgical Endoscopy 2003;17(5):766‐72. - PubMed
Cabral 2008 {published data only}
-
- Cabral PH, Silva IT, Melo JV, Gimenez FS, Cabral CR, Lima AP. Needlescopic versus laparoscopic cholecystectomy. A prospective study of 60 patients. Acta Cirurgica Brasileira 2008;23(6):543‐50. - PubMed
Cheah 2001 {published data only}
-
- Cheah WK, Lenzi JE, So JB, Kum CK, Goh PM. Randomized trial of needlescopic versus laparoscopic cholecystectomy. British Journal of Surgery 2001;88(1):45‐7. - PubMed
Dam 2011 {published data only}
-
- Dam DAV. Single Incision, MiniPort or convEntional Laparoscopic surgery for uncomplicated sympthomatic cholecystolithiasis (SIMPEL‐trial). http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=3100 2011 (accessed on 10th February 2013).
Gupta 2005 {published data only}
-
- Gupta A, Shrivastava UK, Kumar P, Burman D. Minilaparoscopic versus laparoscopic cholecystectomy: a randomised controlled trial. Tropical Gastroenterology 2005;26(3):149‐51. - PubMed
Leggett 2000 {published data only}
-
- Leggett PL, Churchman‐Winn R, Miller G. Minimizing ports to improve laparoscopic cholecystectomy. Surgical Endoscopy 2000;14(1):32‐6. - PubMed
Look 2001 {published data only}
-
- Look M, Chew SP, Tan YC, Liew SE, Cheong DM, Tan JC, et al. Post‐operative pain in needlescopic versus conventional laparoscopic cholecystectomy: a prospective randomised trial. Journal of the Royal College of Surgeons of Edinburgh 2001;46(3):138‐42. - PubMed
Pring 2001 {published data only}
-
- Pring CM. Randomized trial of needlescopic versus laparoscopic cholecystectomy (Comment on: Randomized trial of needlescopic versus laparoscopic cholecystectomy. Cheah WK, Lenzi JE, So JB, Kum CK, Goh PM. Br J Surg. 2001 Jan; 88(1):45‐7). British Journal of Surgery 2001;88(7):1017‐8. - PubMed
Yorganci 2001 {published data only}
-
- Yorganci K, Kaynaroglu ZV, Oner Z. Randomized trial of needlescopic versus laparoscopic cholecystectomy (Comment on: Randomized trial of needlescopic versus laparoscopic cholecystectomy. Cheah WK, Lenzi JE, So JB, Kum CK, Goh PM. Br J Surg. 2001 Jan; 88(1):45‐7). British Journal of Surgery 2001;88(7):1017‐8. - PubMed
Additional references
Bakken 2004
-
- Bakken IJ, Skjeldestad FE, Mjåland O, Johnson E. Cholecystectomy in Norway 1990‐2002 [Kolecystektomi i Norge i 1990‐2002]. Tidsskrift for den Norske Laegeforening 2004;124(18):2376‐8. - PubMed
Brok 2008
-
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61:763‐9. - PubMed
Brok 2009
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive‐‐Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. - PubMed
CTU 2011
-
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. http://ctu.dk/tsa/ 2011 (accessed 5 July 2013).
David 2008
-
- David GG, Al‐Sarira AA, Willmott S, Deakin M, Corless DJ, Slavin JP. Management of acute gallbladder disease in England. British Journal of Surgery 2008;95(4):472‐6. [PUBMED: 17968981] - PubMed
DeMets 1987
-
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Egger 1997
Fullarton 1994
Giger 2011
-
- Giger U, Ouaissi M, Schmitz SF, Krahenbuhl S, Krahenbuhl L. Bile duct injury and use of cholangiography during laparoscopic cholecystectomy. The British Journal of Surgery 2011;98(3):391‐6. - PubMed
Gluud 2013
-
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2013, Issue 6. Art. No.: LIVER.
Gurtner 2008
-
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453(7193):314‐21. - PubMed
Gurusamy 2009
-
- Gurusamy KS, Gluud C, Nikolova D, Davidson BR. Assessment of risk of bias in randomized clinical trials in surgery. British Journal of Surgery 2009;96(4):342‐9. - PubMed
Halldestam 2004
-
- Halldestam I, Enell EL, Kullman E, Borch K. Development of symptoms and complications in individuals with asymptomatic gallstones. British Journal of Surgery 2004;91(6):734‐8. - PubMed
Higgins 2002
-
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Colloboration, 2011. Available from www.cochrane‐handbook.org.
Hosono 2007
-
- Hosono S, Osaka H. Minilaparoscopic versus conventional laparoscopic cholecystectomy: a meta‐analysis of randomized controlled trials. Journal of Laparoendoscopic & Advanced Surgical Techniques. Part A 2007;17(2):191‐9. - PubMed
ICH‐GCP 1997
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997.
Jørgensen 1987
-
- Jørgensen T. Prevalence of gallstones in a Danish population. American Journal of Epidemiology 1987;126(5):912‐21. - PubMed
Keus 2006
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Livingston 2004
-
- Livingston EH, Rege RV. A nationwide study of conversion from laparoscopic to open cholecystectomy. American Journal of Surgery 2004;188(3):205‐11. [PUBMED: 15450821] - PubMed
Lundh 2012
Macaskill 2001
-
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. - PubMed
McCloy 2008
-
- McCloy R, Randall D, Schug SA, Kehlet H, Simanski C, Bonnet F, et al. Is smaller necessarily better? A systematic review comparing the effects of minilaparoscopic and conventional laparoscopic cholecystectomy on patient outcomes. Surgical Endoscopy 2008;22(12):2541‐53. - PubMed
Mjäland 1998
-
- Mjäland O, Adamsen S, Hjelmquist B, Ovaska J, Buanes T. Cholecystectomy rates, gallstone prevalence, and handling of bile duct injuries in Scandinavia. A comparative audit. Surgical Endoscopy 1998;12(12):1386‐9. - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. - PubMed
Muhrbeck 1995
-
- Muhrbeck O, Ahlberg J. Prevalence of gallstone disease in a Swedish population. Scandinavian Journal of Gastroenterology 1995;30(11):1125‐8. - PubMed
Newell 1992
-
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. - PubMed
NIH 1992
-
- National Institutes of Health. Gallstones and Laparoscopic Cholecystectomy, NIH Consens Statement Online 1992 Sep 14‐16. http://consensus.nih.gov/1992/1992GallstonesLaparoscopy090html.htm 1992 (accessed 5 July 2013).
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Savović 2012a
-
- Savović J, Jones H, Altman D, Harris R, Juni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1‐82. - PubMed
Savović 2012b
-
- Savović J, Jones HE, Altman DG, Harris RJ, Juni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. - PubMed
Thakur 2011
-
- Thakur V, Schlachta CM, Jayaraman S. Minilaparoscopic versus conventional laparoscopic cholecystectomy a systematic review and meta‐analysis. Annals of Surgery 2011;253(2):244‐58. - PubMed
Thorlund 2009
-
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. - PubMed
Thorlund 2010
Thorlund 2011
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual forTrial Sequential Analysis (TSA). http://ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 5 July 2013).
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. - PubMed
Wetterslev 2009
References to other published versions of this review
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

