Prolactin promotes cartilage survival and attenuates inflammation in inflammatory arthritis
- PMID: 23908112
- PMCID: PMC3754268
- DOI: 10.1172/JCI69485
Prolactin promotes cartilage survival and attenuates inflammation in inflammatory arthritis
Abstract
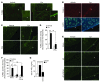
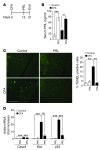
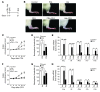
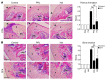
Chondrocytes are the only cells in cartilage, and their death by apoptosis contributes to cartilage loss in inflammatory joint diseases, such as rheumatoid arthritis (RA). A putative therapeutic intervention for RA is the inhibition of apoptosis-mediated cartilage degradation. The hormone prolactin (PRL) frequently increases in the circulation of patients with RA, but the role of hyperprolactinemia in disease activity is unclear. Here, we demonstrate that PRL inhibits the apoptosis of cultured chondrocytes in response to a mixture of proinflammatory cytokines (TNF-α, IL-1β, and IFN-γ) by preventing the induction of p53 and decreasing the BAX/BCL-2 ratio through a NO-independent, JAK2/STAT3-dependent pathway. Local treatment with PRL or increasing PRL circulating levels also prevented chondrocyte apoptosis evoked by injecting cytokines into the knee joints of rats, whereas the proapoptotic effect of cytokines was enhanced in PRL receptor-null (Prlr(-/-)) mice. Moreover, eliciting hyperprolactinemia in rats before or after inducing the adjuvant model of inflammatory arthritis reduced chondrocyte apoptosis, proinflammatory cytokine expression, pannus formation, bone erosion, joint swelling, and pain. These results reveal the protective effect of PRL against inflammation-induced chondrocyte apoptosis and the therapeutic potential of hyperprolactinemia to reduce permanent joint damage and inflammation in RA.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

