Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer
- PMID: 23908119
- PMCID: PMC3726176
- DOI: 10.1172/JCI70212
Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer
Abstract
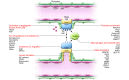
Four decades ago, angiogenesis was recognized as a therapeutic target for blocking cancer growth. Because of its importance, VEGF has been at the center stage of antiangiogenic therapy. Now, several years after FDA approval of an anti-VEGF antibody as the first antiangiogenic agent, many patients with cancer and ocular neovascularization have benefited from VEGF-targeted therapy; however, this anticancer strategy is challenged by insufficient efficacy, intrinsic refractoriness, and resistance. Here, we examine recent discoveries of new mechanisms underlying angiogenesis, discuss successes and challenges of current antiangiogenic therapy, and highlight emerging antiangiogenic paradigms.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

