Pharmacologic inhibition of PKCα and PKCθ prevents GVHD while preserving GVL activity in mice
- PMID: 23908466
- PMCID: PMC3790515
- DOI: 10.1182/blood-2012-12-471938
Pharmacologic inhibition of PKCα and PKCθ prevents GVHD while preserving GVL activity in mice
Abstract
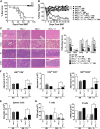
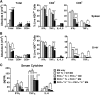
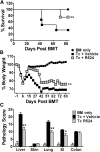
Allogeneic hematopoietic cell transplantation (HCT) is the most effective therapy for hematopoietic malignancies through T-cell-mediated graft-vs-leukemia (GVL) effects but often leads to severe graft-vs-host disease (GVHD). Given that protein kinase Cθ (PKCθ), in cooperation with PKCα, is essential for T-cell signaling and function, we have evaluated PKCθ and PKCα as potential therapeutic targets in allogeneic HCT using genetic and pharmacologic approaches. We found that the ability of PKCα(-/-)/θ(-/-) donor T cells to induce GVHD was further reduced compared with PKCθ(-/-) T cells in relation with the relevance of both isoforms to allogeneic donor T-cell proliferation, cytokine production, and migration to GVHD target organs. Treatment with a specific inhibitor for both PKCθ and PKCα impaired donor T-cell proliferation, migration, and chemokine/cytokine production and significantly decreased GVHD in myeloablative preclinical murine models of allogeneic HCT. Moreover, pharmacologic inhibition of PKCθ and PKCα spared T-cell cytotoxic function and GVL effects. Our findings indicate that PKCα and θ contribute to T-cell activation with overlapping functions essential for GVHD induction while less critical to the GVL effect. Thus, targeting PKCα and PKCθ signaling with pharmacologic inhibitors presents a therapeutic option for GVHD prevention while largely preserving the GVL activity in patients receiving HCT.
Figures




Comment in
-
Doubling down on PKC benefits allogeneic BMT.Blood. 2013 Oct 3;122(14):2298-9. doi: 10.1182/blood-2013-08-519900. Blood. 2013. PMID: 24092926 Free PMC article.
References
-
- Dustin ML. PKC-θ: hitting the bull’s eye. Nat Immunol. 2011;12(11):1031–1032. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

