Host matrix modulation by tumor exosomes promotes motility and invasiveness
- PMID: 23908589
- PMCID: PMC3730040
- DOI: 10.1593/neo.13786
Host matrix modulation by tumor exosomes promotes motility and invasiveness
Abstract
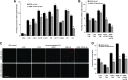
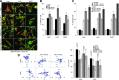
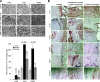
Exosomes are important intercellular communicators, where tumor exosomes (TEX) severely influence hematopoiesis and premetastatic organ cells. With the extracellular matrix (ECM) being an essential constituent of non-transformed tissues and tumors, we asked whether exosomes from a metastatic rat tumor also affect the organization of the ECM and whether this has consequences on host and tumor cell motility. TEX bind to individual components of the ECM, the preferential partner depending on the exosomes' adhesion molecule profile such that high CD44 expression is accompanied by hyaluronic acid binding and high α6β4 expression by laminin (LN) 332 binding, which findings were confirmed by antibody blocking. TEX can bind to the tumor matrix already during exosome delivery but also come in contact with distinct organ matrices. Being rich in proteases, TEX modulate the ECM as demonstrated for degradation of collagens, LNs, and fibronectin. Matrix degradation by TEX has severe consequences on tumor and host cell adhesion, motility, and invasiveness. By ECM degradation, TEX also promote host cell proliferation and apoptosis resistance. Taken together, the host tissue ECM modulation by TEX is an important factor in the cross talk between a tumor and the host including premetastatic niche preparation and the recruitment of hematopoietic cells. Reorganization of the ECM by exosomes likely also contributes to organogenesis, physiological and pathologic angiogenesis, wound healing, and clotting after vessel disruption.
Figures





References
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
-
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. - PubMed
-
- Stewart TJ, Smyth MJ. Improving cancer immunotherapy by targeting tumor-induced immune suppression. Cancer Metastasis Rev. 2011;30:125–140. - PubMed
-
- Brabletz T. EMT and MET in metastasis: where are the cancer stem cells? Cancer Cell. 2012;22:699–701. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
