Flavopiridol synergizes with sorafenib to induce cytotoxicity and potentiate antitumorigenic activity in EGFR/HER-2 and mutant RAS/RAF breast cancer model systems
- PMID: 23908594
- PMCID: PMC3730045
- DOI: 10.1593/neo.13804
Flavopiridol synergizes with sorafenib to induce cytotoxicity and potentiate antitumorigenic activity in EGFR/HER-2 and mutant RAS/RAF breast cancer model systems
Abstract
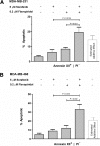
Oncogenic receptor tyrosine kinase (RTK) signaling through the Ras-Raf-Mek-Erk (Ras-MAPK) pathway is implicated in a wide array of carcinomas, including those of the breast. The cyclin-dependent kinases (CDKs) are implicated in regulating proliferative and survival signaling downstream of this pathway. Here, we show that CDK inhibitors exhibit an order of magnitude greater cytotoxic potency than a suite of inhibitors targeting RTK and Ras-MAPK signaling in cell lines representative of clinically recognized breast cancer (BC) subtypes. Drug combination studies show that the pan-CDK inhibitor, flavopiridol (FPD), synergistically potentiated cytotoxicity induced by the Raf inhibitor, sorafenib (SFN). This synergy was most pronounced at sub-EC50 SFN concentrations in MDA-MB-231 (KRAS-G13D and BRAF-G464V mutations), MDA-MB-468 [epidermal growth factor receptor (EGFR) overexpression], and SKBR3 [ErbB2/EGFR2 (HER-2) overexpression] cells but not in hormone-dependent MCF-7 and T47D cells. Potentiation of SFN cytotoxicity by FPD correlated with enhanced apoptosis, suppression of retinoblastoma (Rb) signaling, and reduced Mcl-1 expression. SFN and FPD were also tested in an MDA-MB-231 mammary fat pad engraftment model of tumorigenesis. Mice treated with both drugs exhibited reduced primary tumor growth rates and metastatic tumor load in the lungs compared to treatment with either drug alone, and this correlated with greater reductions in Rb signaling and Mcl-1 expression in resected tumors. These findings support the development of CDK and Raf co-targeting strategies in EGFR/HER-2-overexpressing or RAS/RAF mutant BCs.
Figures






References
-
- Rusnak DW, Alligood KJ, Mullin RJ, Spehar GM, Arenas-Elliott C, Martin AM, Degenhardt Y, Rudolph SK, Haws TF, Jr, Hudson-Curtis BL, et al. Assessment of epidermal growth factor receptor (EGFR, ErbB1) and HER2 (ErbB2) protein expression levels and response to lapatinib (Tykerb, GW572016) in an expanded panel of human normal and tumour cell lines. Cell Prolif. 2007;40:580–594. - PMC - PubMed
-
- Pegram MD, Konecny GE, O'Callaghan C, Beryt M, Pietras R, Slamon DJ. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst. 2004;96:739–749. - PubMed
-
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al. Studies of the HER-2/neu protooncogene in human breast and ovarian cancer. Science. 1989;244:707–712. - PubMed
-
- Janne PA, Gray N, Settleman J. Factors underlying sensitivity of cancers to small-molecule kinase inhibitors. Nat Rev Drug Discov. 2009;8:709–723. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
