Issues related to development of antiepileptogenic therapies
- PMID: 23909852
- PMCID: PMC3740390
- DOI: 10.1111/epi.12297
Issues related to development of antiepileptogenic therapies
Abstract
Several preclinical proof-of-concept studies have provided evidence for positive treatment effects on epileptogenesis. However, none of these hypothetical treatments has advanced to the clinic. The experience in other fields of neurology such as stroke, Alzheimer's disease, or amyotrophic lateral sclerosis has indicated several problems in the design of preclinical studies, which likely contribute to failures in translating the positive preclinical data to the clinic. The Working Group on "Issues related to development of antiepileptogenic therapies" of the International League Against Epilepsy (ILAE) and the American Epilepsy Society (AES) has considered the possible problems that arise when moving from proof-of-concept antiepileptogenesis (AEG) studies to preclinical AEG trials, and eventually to clinical AEG trials. This article summarizes the discussions and provides recommendations on how to design a preclinical AEG monotherapy trial in adult animals. We specifically address study design, animal and model selection, number of studies needed, issues related to administration of the treatment, outcome measures, statistics, and reporting. In addition, we give recommendations for future actions to advance the preclinical AEG testing.
Keywords: Disease modification; Epilepsy; Epileptogenesis; Preclinical; Protocol; Therapy.
Wiley Periodicals, Inc. © 2013 International League Against Epilepsy.
Figures
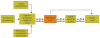

References
-
- Ali A, Dua Y, Constance JE, Franklin MR, Dudek FE. A once-per-day, drug-in-food protocol for prolonged administration of antiepileptic drugs in animal models. Epilepsia. 2012;53(1):199–206. - PubMed
-
- Brooks-Kayal A, Bath K, Berg A, Galanopoulou A, Holmes G, Jensen F, Kanner A, O’Brien T, Patel M, Scharfman H, Whittemore V, Winawer M. Issues related to symptomatic and disease modifying treatments affecting cognitive and neuropsychiatric comorbidities of epilepsy. Epilepsia. 2013 submitted. - PMC - PubMed
-
- Fisher RS, Blum DE, DiVentura B, Vannest J, Hixson JD, Moss R, Herman ST, Fureman BE, French JA. Seizure diaries for clinical research and practice: limitations and future prospects. Epilepsy Behav. 2012;24(3):304–310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS038595/NS/NINDS NIH HHS/United States
- R01 NS044370/NS/NINDS NIH HHS/United States
- DP1 OD003347/OD/NIH HHS/United States
- R01 NS040337/NS/NINDS NIH HHS/United States
- R01 NS020253/NS/NINDS NIH HHS/United States
- R21 NS079135/NS/NINDS NIH HHS/United States
- R01 NS079274/NS/NINDS NIH HHS/United States
- R25 NS070682/NS/NINDS NIH HHS/United States
- RC1 NS068938/NS/NINDS NIH HHS/United States
- R21 NS049525/NS/NINDS NIH HHS/United States
- R01 NS075429/NS/NINDS NIH HHS/United States
- R25 NS065733/NS/NINDS NIH HHS/United States
- R44 NS064661/NS/NINDS NIH HHS/United States
- R21 NS072258/NS/NINDS NIH HHS/United States
- R01 NS030549/NS/NINDS NIH HHS/United States
- R01 NS031718/NS/NINDS NIH HHS/United States
- R01 NS045144/NS/NINDS NIH HHS/United States
- N01 NS042359/NS/NINDS NIH HHS/United States
- R56 NS020253/NS/NINDS NIH HHS/United States
- R21 NS078333/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

