Epilepsy biomarkers
- PMID: 23909854
- PMCID: PMC4131763
- DOI: 10.1111/epi.12299
Epilepsy biomarkers
Abstract
A biomarker is defined as an objectively measured characteristic of a normal or pathologic biologic process. Identification and proper validation of biomarkers of epileptogenesis (the development of epilepsy) and ictogenesis (the propensity to generate spontaneous seizures) might predict the development of an epilepsy condition; identify the presence and severity of tissue capable of generating spontaneous seizures; measure progression after the condition is established; and determine pharmacoresistance. Such biomarkers could be used to create animal models for more cost-effective screening of potential antiepileptogenic and antiseizure drugs and devices, and to reduce the cost of clinical trials by enriching the trial population, and acting as surrogate markers to shorten trial duration. The objectives of the biomarker subgroup for the London Workshop were to define approaches for identifying possible biomarkers for these purposes. Research to identify reliable biomarkers may also reveal underlying mechanisms that could serve as therapeutic targets for the development of new antiepileptogenic and antiseizure compounds.
Keywords: Biomarkers; Epileptogenesis; Ictogenesis; Surrogate markers; Therapeutic intervention.
Wiley Periodicals, Inc. © 2013 International League Against Epilepsy.
Figures

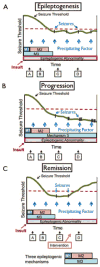
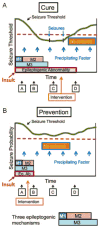
References
-
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, Engel J, Jr, French J, Glauser TA, Mathern GW, Moshé SL, Nordli D, Jr, Plouin P, Scheffer IE. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. - PubMed
-
- Engel J., Jr . Seizures and Epilepsy. Philadelphia: F. A. Davis; 1989. p. 536.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

