Performance evaluations of continuous glucose monitoring systems: precision absolute relative deviation is part of the assessment
- PMID: 23911163
- PMCID: PMC3879746
- DOI: 10.1177/193229681300700404
Performance evaluations of continuous glucose monitoring systems: precision absolute relative deviation is part of the assessment
Abstract
Background: Even though a Clinical and Laboratory Standards Institute proposal exists on the design of studies and performance criteria for continuous glucose monitoring (CGM) systems, it has not yet led to a consistent evaluation of different systems, as no consensus has been reached on the reference method to evaluate them or on acceptance levels. As a consequence, performance assessment of CGM systems tends to be inconclusive, and a comparison of the outcome of different studies is difficult.
Materials and methods: Published information and available data (as presented in this issue of Journal of Diabetes Science and Technology by Freckmann and coauthors) are used to assess the suitability of several frequently used methods [International Organization for Standardization, continuous glucose error grid analysis, mean absolute relative deviation (MARD), precision absolute relative deviation (PARD)] when assessing performance of CGM systems in terms of accuracy and precision.
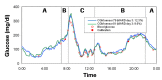
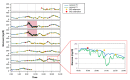
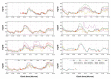
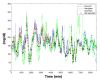
Results: The combined use of MARD and PARD seems to allow for better characterization of sensor performance. The use of different quantities for calibration and evaluation, e.g., capillary blood using a blood glucose (BG) meter versus venous blood using a laboratory measurement, introduces an additional error source. Using BG values measured in more or less large intervals as the only reference leads to a significant loss of information in comparison with the continuous sensor signal and possibly to an erroneous estimation of sensor performance during swings. Both can be improved using data from two identical CGM sensors worn by the same patient in parallel.
Conclusions: Evaluation of CGM performance studies should follow an identical study design, including sufficient swings in glycemia. At least a part of the study participants should wear two identical CGM sensors in parallel. All data available should be used for evaluation, both by MARD and PARD, a good PARD value being a precondition to trust a good MARD value. Results should be analyzed and presented separately for clinically different categories, e.g., hypoglycemia, exercise, or night and day.
© 2013 Diabetes Technology Society.
Figures



References
-
- International Organization for Standardization. ISO 15197:2013. In vitro diagnostic test systems -- requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. http://www.iso.org/iso/home/store/catalogue_tc/catalogue_detail.htm?csnu....
-
- Clinical and Laboratory Standards Institute. Performance metrics for continuous interstitial glucose monitoring; approved guideline. http://shopping.netsuite.com/c.1253739/site/Sample_pdf/POCT05A__sample.pdf.
-
- Kovatchev BP, Gonder-Frederick LA, Cox DJ, Clarke WL. Evaluating the accuracy of continuous glucose-monitoring sensors: continuous glucose-error grid analysis illustrated by TheraSense Freestyle Navigator data. Diabetes Care. 2004;27(8):1922–1928. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

