Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer
- PMID: 23911236
- PMCID: PMC3753022
- DOI: 10.1016/j.ccr.2013.06.014
Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer
Erratum in
- Cancer Cell. 2013 Sep 9;24(3):399
Abstract
Accelerated glucose metabolism is a common feature of cancer cells. Hexokinases catalyze the first committed step of glucose metabolism. Hexokinase 2 (HK2) is expressed at high level in cancer cells, but only in a limited number of normal adult tissues. Using Hk2 conditional knockout mice, we showed that HK2 is required for tumor initiation and maintenance in mouse models of KRas-driven lung cancer, and ErbB2-driven breast cancer, despite continued HK1 expression. Similarly, HK2 ablation inhibits the neoplastic phenotype of human lung and breast cancer cells in vitro and in vivo. Systemic Hk2 deletion is therapeutic in mice bearing lung tumors without adverse physiological consequences. Hk2 deletion in lung cancer cells suppressed glucose-derived ribonucleotides and impaired glutamine-derived carbon utilization in anaplerosis.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
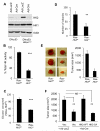
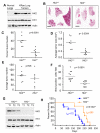

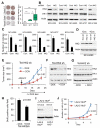
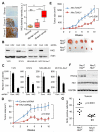


References
-
- Felig P, Wahren J. Fuel homeostasis in exercise. N Engl J Med. 1975;293:1078–1084. - PubMed
-
- Fueger PT, Heikkinen S, Bracy DP, Malabanan CM, Pencek RR, Laakso M, Wasserman DH. Hexokinase II partial knockout impairs exercise-stimulated glucose uptake in oxidative muscles of mice. Am J Physiol Endocrinol Metab. 2003;285:E958–963. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
