Coronary adventitial cells are linked to perivascular cardiac fibrosis via TGFβ1 signaling in the mdx mouse model of Duchenne muscular dystrophy
- PMID: 23911435
- PMCID: PMC3834000
- DOI: 10.1016/j.yjmcc.2013.07.014
Coronary adventitial cells are linked to perivascular cardiac fibrosis via TGFβ1 signaling in the mdx mouse model of Duchenne muscular dystrophy
Abstract
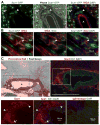
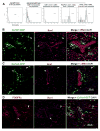
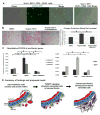
In Duchenne muscular dystrophy (DMD), progressive accumulation of cardiac fibrosis promotes heart failure. While the cellular origins of fibrosis in DMD hearts remain enigmatic, fibrotic tissue conspicuously forms near the coronary adventitia. Therefore, we sought to characterize the role of coronary adventitial cells in the formation of perivascular fibrosis. Utilizing the mdx model of DMD, we have identified a population of Sca1+, PDGFRα+, CD31-, and CD45- coronary adventitial cells responsible for perivascular fibrosis. Histopathology of dystrophic hearts revealed that Sca1+ cells extend from the adventitia and occupy regions of perivascular fibrosis. The number of Sca1+ adventitial cells increased two-fold in fibrotic mdx hearts vs. age matched wild-type hearts. Moreover, relative to Sca1-, PDGFRα+, CD31-, and CD45- cells and endothelial cells, Sca1+ adventitial cells FACS-sorted from mdx hearts expressed the highest level of Collagen1α1 and 3α1, Connective tissue growth factor, and Tgfβr1 transcripts. Surprisingly, mdx endothelial cells expressed the greatest level of the Tgfβ1 ligand. Utilizing Collagen1α1-GFP reporter mice, we confirmed that the majority of Sca1+ adventitial cells expressed type I collagen, an abundant component of cardiac fibrosis, in both wt (71%±4.1) and mdx (77%±3.5) hearts. In contrast, GFP+ interstitial fibroblasts were PDGFRα+ but negative for Sca1. Treatment of cultured Collagen1α1-GFP+ adventitial cells with TGFβ1 resulted in increased collagen synthesis, whereas pharmacological inhibition of TGFβR1 signaling reduced the fibrotic response. Therefore, perivascular cardiac fibrosis by coronary adventitial cells may be mediated by TGFβ1 signaling. Our results implicate coronary endothelial cells in mediating cardiac fibrosis via transmural TGFβ signaling, and suggest that the coronary adventitia is a promising target for developing novel anti-fibrotic therapies.
Keywords: Adventitia; Fibrosis; Muscular dystrophy; Perivascular; Sca1; TGFβ1; Type I collagen.
© 2013.
Conflict of interest statement
The authors concede no conflicts of interest in the publication of this manuscript.
Figures



References
-
- Deconinck N, Dan B. Pathophysiology of duchenne muscular dystrophy: current hypotheses. Pediatric neurology. 2007;36:1–7. - PubMed
-
- Culligan KG, Mackey AJ, Finn DM, Maguire PB, Ohlendieck K. Role of dystrophin isoforms and associated proteins in muscular dystrophy (review) International journal of molecular medicine. 1998;2:639–48. - PubMed
-
- Sultan A, Fayaz M. Prevalence of cardiomyopathy in Duchenne and Becker’s muscular dystrophy. J Ayub Med Coll Abbottabad. 2008;20:7–13. - PubMed
-
- Spurney CF. Cardiomyopathy of Duchenne muscular dystrophy: current understanding and future directions. Muscle & nerve. 2011;44:8–19. - PubMed
-
- Eagle M, Baudouin SV, Chandler C, Giddings DR, Bullock R, Bushby K. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord. 2002;12:926–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

