Comparison of placebo and best available therapy for the treatment of myelofibrosis in the phase 3 COMFORT studies
- PMID: 23911705
- PMCID: PMC3912959
- DOI: 10.3324/haematol.2013.087650
Comparison of placebo and best available therapy for the treatment of myelofibrosis in the phase 3 COMFORT studies
Abstract
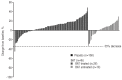
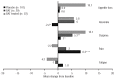
Prior to Janus kinase inhibitors, available therapies for myelofibrosis were generally supportive and did not improve survival. This analysis compares efficacy outcomes of patients with myelofibrosis in the control arms (placebo [n=154] and best available therapy [n=73]) from the two phase 3 COntrolled MyeloFibrosis study with ORal JAK inhibitor Treatment (COMFORT) studies. Spleen volume was assessed by magnetic resonance imaging/computed tomography at baseline and every 12 weeks through week 72; spleen length was assessed by palpation at each study visit. Health-related quality of life and symptoms were assessed using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 Items at baseline and in weeks 4, 8, 12, 16 and 24 in COMFORT-I and in weeks 8, 16, 24 and 48 in COMFORT-II. The demographic and baseline characteristics were similar between the control arms of the two studies. One patient who received placebo and no patients who received best available therapy had a ≥35% reduction in spleen volume from baseline at week 24. At 24 weeks, neither placebo nor best available therapy had produced clinically meaningful changes in global quality of life or symptom scales. Non-hematologic adverse events were mostly grade 1/2; the most frequently reported adverse events in each group were abdominal pain, fatigue, peripheral edema and diarrhea. These data suggest that non-Janus kinase inhibitor therapies provide little improvement in splenomegaly, symptoms or quality of life as compared with placebo. Both COMFORT-I (NCT00952289) and COMFORT-II (NCT00934544) studies have been appropriately registered with clinicaltrials.gov.
Figures


References
-
- Mesa RA, Verstovsek S, Cervantes F, Barosi G, Reilly JT, Dupriez B, et al. Primary myelofibrosis (PMF), post polycythemia vera myelofibrosis (post-PV MF), post essential thrombocythemia myelofibrosis (post-ET MF), blast phase PMF (PMF-BP): consensus on terminology by the International Working Group for Myelofibrosis Research and Treatment (IWG-MRT). Leuk Res. 2007;31(6):737–40 - PubMed
-
- Cervantes F, Passamonti F, Barosi G. Life expectancy and prognostic factors in the classic BCR/ABL-negative myeloproliferative disorders. Leukemia. 2008;22(5):905–14 - PubMed
-
- Mesa RA, Niblack J, Wadleigh M, Verstovsek S, Camoriano J, Barnes S, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer. 2007;109(1):68–76 - PubMed
-
- Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895–901 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

