Phoenixin: a novel peptide in rodent sensory ganglia
- PMID: 23912037
- PMCID: PMC3775297
- DOI: 10.1016/j.neuroscience.2013.07.057
Phoenixin: a novel peptide in rodent sensory ganglia
Abstract
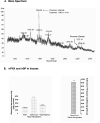
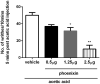
Phoenixin-14 amide, herein referred to as phoenixin, is a newly identified peptide from the rat brain. Using a previously characterized rabbit polyclonal antiserum against phoenixin, enzyme-immunoassay detected a high level (>4.5 ng/g tissue) of phoenixin-immunoreactivity (irPNX) in the rat spinal cords. Immunohistochemical studies revealed irPNX in networks of cell processes in the superficial dorsal horn, spinal trigeminal tract and nucleus of the solitary tract; and in a population of dorsal root, trigeminal and nodose ganglion cells. The pattern of distribution of irPNX in the superficial layers of the dorsal horn was similar to that of substance P immunoreactivity (irSP). Double-labeling the dorsal root ganglion sections showed that irPNX and irSP express in different populations of ganglion cells. In awake mice, intrathecal injection of phoenixin (1 or 5 μg) did not significantly affect the tail-flick latency as compared to that in animals injected with artificial cerebrospinal fluid (aCSF). Intrathecal administration of phoenixin (0.5, 1.25 or 2.5 μg) significantly reduced the number of writhes elicited by intraperitoneal injection of acetic acid (0.6%, 0.3 ml/30 g) as compared to that in mice injected with aCSF. While not affecting the tail-flick latency, phoenixin antiserum (1:100) injected intrathecally 10 min prior to the intraperitoneal injection of acetic acid significantly increased the number of writhes as compared to mice pre-treated with normal rabbit serum. Intrathecal injection of non-amidated phoenixin (2.5 μg) did not significantly alter the number of writhes evoked by acetic acid. Our result shows that phoenixin is expressed in sensory neurons of the dorsal root, nodose and trigeminal ganglia, the amidated peptide is bioactive, and exogenously administered phoenixin may preferentially suppress visceral as opposed to thermal pain.
Keywords: ABC; ANOVA; BCA; DRG; EIA; FITC; G-protein-coupled receptor; GPCR; MALDI-TOF; PBS; PEPS; PNX; RP-HPLC; SDB-L; aCSF; analysis of variance; artificial cerebrospinal fluid; avidin–biotin complex; bicinchoninic acid protein assay; dorsal root ganglia; enzyme-immunoassay; fluorescein isothiocyanate; irPNX; irSP; matrix-assisted laser desorption/ionization time-of-flight mass spectrometry; nAmb; nucleus ambiguus; pericentral spikes; phoenixin immunoreactivity; phoenixin-14 amide; phosphate-buffered saline.; reverse-phase High-performance liquid chromatography; sensory neurons; spinal cord; styrene-divinylbenzene polymer; substance P immunoreactivity; thermal pain; visceral pain.
Copyright © 2013 IBRO. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures






References
-
- Cervero F, Connell LA. Distribution of somatic and visceral primary afferent fibres within the thoracic spinal cord of the cat. J Comp Neurol. 1984;230:88–98. - PubMed
-
- Cheng Z, Powley TL. Nucleus ambiguus projections to cardiac ganglia of rat atria: an anterograde tracing study. J Comp Neurol. 2000;424:588–606. - PubMed
-
- Chuang YC, Yoshimura N, Huang CC, Chiang PH, Chancellor MB. Intravesical botulinum toxin administration produces analgesia against acetic acid induced bladder pain responses in rats. J Urol. 2004;172:1529–1532. - PubMed
-
- DeLeo JA, Coombs DW, McCarthy LE. Differential c-fos-like protein expression in mechanically versus chemically induced visceral nociception. Brain Res Mol Brain Res. 1991;1:167–170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

