Magnetic iron oxide nanoparticles for multimodal imaging and therapy of cancer
- PMID: 23912234
- PMCID: PMC3759893
- DOI: 10.3390/ijms140815910
Magnetic iron oxide nanoparticles for multimodal imaging and therapy of cancer
Abstract
Superparamagnetic iron oxide nanoparticles (SPION) have emerged as an MRI contrast agent for tumor imaging due to their efficacy and safety. Their utility has been proven in clinical applications with a series of marketed SPION-based contrast agents. Extensive research has been performed to study various strategies that could improve SPION by tailoring the surface chemistry and by applying additional therapeutic functionality. Research into the dual-modal contrast uses of SPION has developed because these applications can save time and effort by reducing the number of imaging sessions. In addition to multimodal strategies, efforts have been made to develop multifunctional nanoparticles that carry both diagnostic and therapeutic cargos specifically for cancer. This review provides an overview of recent advances in multimodality imaging agents and focuses on iron oxide based nanoparticles and their theranostic applications for cancer. Furthermore, we discuss the physiochemical properties and compare different synthesis methods of SPION for the development of multimodal contrast agents.
Figures
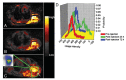
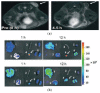
References
-
- Na H.B., Song I.C., Hyeon T. Inorganic nanoparticles for mri contrast agents. Adv. Mater. 2009;21:2133–2148.
-
- Willmann J.K., van Bruggen N., Dinkelborg L.M., Gambhir S.S. Molecular imaging in drug development. Nat. Rev. Drug Discov. 2008;7:591–607. - PubMed
-
- Kirui D.K., Khalidov I., Wang Y., Batt C.A. Targeted near-ir hybrid magnetic nanoparticles for in vivo cancer therapy and imaging. Nanomed. Nanotechnol. Biol. Med. 2012;9:702–711. - PubMed
-
- Misri R., Meier D., Yung A.C., Kozlowski P., Hafeli U.O. Development and evaluation of a dual-modality (mri/spect) molecular imaging bioprobe. Nanomed. Nanotechnol. Biol. Med. 2012;8:1007–1016. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

