Effect of δ-opioid receptor activation on BDNF-TrkB vs. TNF-α in the mouse cortex exposed to prolonged hypoxia
- PMID: 23912236
- PMCID: PMC3759895
- DOI: 10.3390/ijms140815959
Effect of δ-opioid receptor activation on BDNF-TrkB vs. TNF-α in the mouse cortex exposed to prolonged hypoxia
Abstract
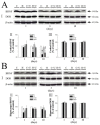
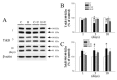
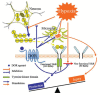
We investigated whether δ-opioid receptor (DOR)-induced neuroprotection involves the brain-derived neurotrophic factor (BDNF) pathway. We studied the effect of DOR activation on the expression of BDNF and other proteins in the cortex of C57BL/6 mice exposed to hypoxia (10% of oxygen) for 1-10 days. The results showed that: (1) 1-day hypoxia had no appreciable effect on BDNF expression, while 3- and 10-day hypoxia progressively decreased BDNF expression, resulting in 37.3% reduction (p < 0.05) after 10-day exposure; (2) DOR activation with UFP-512 (1 mg/kg, i.p., daily) partially reversed the hypoxia-induced reduction of BDNF expression in the 3- or 10-day exposed cortex; (3) DOR activation partially reversed the hypoxia-induced reduction in functional TrkB (140-kDa) and attenuated hypoxia-induced increase in truncated TrkB (90-kDa) in the 3- or 10-day hypoxic cortex; and (4) prolonged hypoxia (10 days) significantly increased TNF-α level and decreased CD11b expression in the cortex, which was completely reversed following DOR activation; and (5) there was no significant change in pCREB and pATF-1 levels in the hypoxic cortex. We conclude that prolonged hypoxia down-regulates BDNF-TrkB signaling leading to an increase in TNF-α in the cortex, while DOR activation up-regulates BDNF-TrkB signaling thereby decreasing TNF-α levels in the hypoxic cortex.
Figures




References
-
- Zhang J., Haddad G.G., Xia Y. Delta-, but not mu- and kappa-, opioid receptor activation protects neocortical neurons from glutamate-induced excitotoxic injury. Brain Res. 2000;885:143–153. - PubMed
-
- Tian X.S., Zhou F., Yang R., Xia Y., Wu G.C., Guo J.C. Effects of intracerebroventricular injection of delta-opioid receptor agonist TAN-67 or antagonist naltrindole on acute cerebral ischemia in rat. Sheng Li Xue Bao. 2008;60:475–484. - PubMed
-
- Zhao P., Huang Y., Zuo Z. Opioid preconditioning induces opioid receptor-dependent delayed neuroprotection against ischemia in rats. J. Neuropathol. Exp. Neurol. 2006;65:945–952. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

