Neuronal sirtuin1 mediates retinal vascular regeneration in oxygen-induced ischemic retinopathy
- PMID: 23912262
- PMCID: PMC4006695
- DOI: 10.1007/s10456-013-9374-5
Neuronal sirtuin1 mediates retinal vascular regeneration in oxygen-induced ischemic retinopathy
Abstract
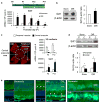
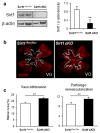
Regeneration of blood vessels in ischemic neuronal tissue is critical to reduce tissue damage in diseases. In proliferative retinopathy, initial vessel loss leads to retinal ischemia, which can induce either regrowth of vessels to restore normal metabolism and minimize damage, or progress to hypoxia-induced sight-threatening pathologic vaso-proliferation. It is not well understood how retinal neurons mediate regeneration of vascular growth in response to ischemic insults. In this study we aim to investigate the potential role of Sirtuin 1 (Sirt1), a metabolically-regulated protein deacetylase, in mediating the response of ischemic neurons to regulate vascular regrowth in a mouse model of oxygen-induced ischemic retinopathy (OIR). We found that Sirt1 is highly induced in the avascular ischemic retina in OIR. Conditional depletion of neuronal Sirt1 leads to significantly decreased retinal vascular regeneration into the avascular zone and increased hypoxia-induced pathologic vascular growth. This effect is likely independent of PGC-1α, a known Sirt1 target, as absence of PGC-1α in knockout mice does not impact vascular growth in retinopathy. We found that neuronal Sirt1 controls vascular regrowth in part through modulating deacetylation and stability of hypoxia-induced factor 1α and 2α, and thereby modulating expression of angiogenic factors. These results indicate that ischemic neurons induce Sirt1 to promote revascularization into ischemic neuronal areas, suggesting a novel role of neuronal Sirt1 in mediating vascular regeneration in ischemic conditions, with potential implications beyond retinopathy.
Conflict of interest statement
Figures


References
-
- Antonetti DA, et al. Diabetic retinopathy. N Engl J Med. 2012;366:1227–1239. - PubMed
-
- Sapieha P. Eyeing central neurons in vascular growth and reparative angiogenesis. Blood. 2012;120:2182–2194. - PubMed
-
- Sapieha P, et al. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat Med. 2008;14:1067–1076. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

