Deletion of TOP3β, a component of FMRP-containing mRNPs, contributes to neurodevelopmental disorders
- PMID: 23912948
- PMCID: PMC3986889
- DOI: 10.1038/nn.3484
Deletion of TOP3β, a component of FMRP-containing mRNPs, contributes to neurodevelopmental disorders
Abstract
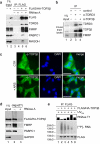
Implicating particular genes in the generation of complex brain and behavior phenotypes requires multiple lines of evidence. The rarity of most high-impact genetic variants typically precludes the possibility of accruing statistical evidence that they are associated with a given trait. We found that the enrichment of a rare chromosome 22q11.22 deletion in a recently expanded Northern Finnish sub-isolate enabled the detection of association between TOP3B and both schizophrenia and cognitive impairment. Biochemical analysis of TOP3β revealed that this topoisomerase was a component of cytosolic messenger ribonucleoproteins (mRNPs) and was catalytically active on RNA. The recruitment of TOP3β to mRNPs was independent of RNA cis-elements and was coupled to the co-recruitment of FMRP, the disease gene product in fragile X mental retardation syndrome. Our results indicate a previously unknown role for TOP3β in mRNA metabolism and suggest that it is involved in neurodevelopmental disorders.
Figures





Comment in
-
The Top3β way to untangle RNA.Nat Neurosci. 2013 Sep;16(9):1163-4. doi: 10.1038/nn.3506. Nat Neurosci. 2013. PMID: 23982446 No abstract available.
-
Genetics: RNA topoisomerase involved in neurodevelopmental disorders.Nat Rev Neurol. 2013 Oct;9(10):542. doi: 10.1038/nrneurol.2013.178. Epub 2013 Sep 10. Nat Rev Neurol. 2013. PMID: 24018476 No abstract available.
References
-
- Peltonen L, Jalanko A, Varilo T. Molecular genetics of the Finnish disease heritage. Hum Mol Genet. 1999;8:1913–1923. - PubMed
-
- Perala J, et al. Geographic variation and sociodemographic characteristics of psychotic disorders in Finland. Schizophr Res. 2008;106:337–347. - PubMed
-
- Palmgren K. Kehittyneisyyden alueittaista eroavuuksista Suomessa (Regional differences in the degree of development in Finland) 1964. (Publications of the National Plannig Bureau A15).
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

