Polypyrrole hollow microspheres as echogenic photothermal agent for ultrasound imaging guided tumor ablation
- PMID: 23912977
- PMCID: PMC3733053
- DOI: 10.1038/srep02360
Polypyrrole hollow microspheres as echogenic photothermal agent for ultrasound imaging guided tumor ablation
Abstract
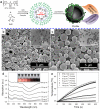
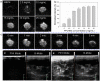
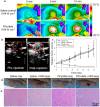
Ultrasound (US) imaging provides a valuable opportunity to administer photothermal therapy (PTT) of cancer with real-time guidance to ensure proper targeting, but only a few theranostic agents were developed by physically grafting near infrared (NIR)-absorbing inorganic nanomaterials to ready-made ultrasound contrast agents (UCAs) for US imaging guided PTT. In this paper, NIR absorbing hollow microspheres were generated from polypyrrole merely using a facile one-step microemulsion method. It was found that the obtained polypyrrole hollow microspheres (PPyHMs) can act as an efficient theranostic agent not only to enhance US imaging greatly, but also exhibit excellent photohyperthermic effects. The contrast consistently sustained the echo signals for no less than 5 min and the NIR laser light ablated the tumor completely within two weeks in the presence of PPyHMs. More importantly, no use of additional NIR absorber substantially minimizes an onetime dose of the theranostic agent.
Figures

References
-
- Vogel A. & Venugopalan V. Mechanisms of pulsed laser ablation of biological tissues. Chem. Rev. 103, 577–644 (2003). - PubMed
-
- Fiedler V. U. et al. Laser-induced interstitial thermotherapy of liver metastases in an interventional 0.5 tesla MRI system: Technique and first clinical experiences. J. Magn. Reson. Imaging 13, 729–737 (2001). - PubMed
-
- Yang K. et al. Multimodal imaging guided photothermal therapy using functionalized graphene nanosheets anchored with magnetic nanoparticles. Adv. Mater. 24, 1868–1872 (2012). - PubMed
-
- Yu M. K. et al. Image-guided prostate cancer therapy using aptamer-functionalized thermally cross-linked superparamagnetic iron oxide nanoparticles. Small 7, 2241–2249 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

