Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism
- PMID: 23913001
- PMCID: PMC3876926
- DOI: 10.1038/ng.2695
Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism
Abstract
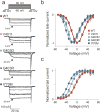
Adrenal aldosterone-producing adenomas (APAs) constitutively produce the salt-retaining hormone aldosterone and are a common cause of severe hypertension. Recurrent mutations in the potassium channel gene KCNJ5 that result in cell depolarization and Ca(2+) influx cause ∼40% of these tumors. We identified 5 somatic mutations (4 altering Gly403 and 1 altering Ile770) in CACNA1D, encoding a voltage-gated calcium channel, among 43 APAs without mutated KCNJ5. The altered residues lie in the S6 segments that line the channel pore. Both alterations result in channel activation at less depolarized potentials; Gly403 alterations also impair channel inactivation. These effects are inferred to cause increased Ca(2+) influx, which is a sufficient stimulus for aldosterone production and cell proliferation in adrenal glomerulosa. We also identified de novo germline mutations at identical positions in two children with a previously undescribed syndrome featuring primary aldosteronism and neuromuscular abnormalities. These findings implicate gain-of-function Ca(2+) channel mutations in APAs and primary aldosteronism.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Spat A, Hunyady L. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol Rev. 2004;84:489–539. - PubMed
-
- Rossi GP, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–300. - PubMed
-
- Conn JW. Presidential address. I Painting background II Primary aldosteronism, a new clinical syndrome. J Lab Clin Med. 1955;45:3–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

