Ritonavir and efavirenz significantly alter the metabolism of erlotinib--an observation in primary cultures of human hepatocytes that is relevant to HIV patients with cancer
- PMID: 23913028
- PMCID: PMC3781374
- DOI: 10.1124/dmd.113.052100
Ritonavir and efavirenz significantly alter the metabolism of erlotinib--an observation in primary cultures of human hepatocytes that is relevant to HIV patients with cancer
Abstract
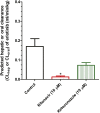
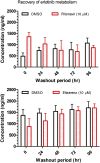
Erlotinib is approved for the treatment of non-small cell lung and pancreatic cancers, and is metabolized by CYP3A4. Inducers and inhibitors of CYP3A enzymes such as ritonavir and efavirenz, respectively, may be used as part of the highly active antiretroviral therapy drugs to treat patients with human immunodeficiency virus (HIV). When HIV patients with a malignancy need treatment with erlotinib, there is a potential of as-yet-undefined drug-drug interaction. We evaluated these interactions using human hepatocytes benchmarked against the interaction of erlotinib with ketoconazole and rifampin, the archetype cytochrome P450 inhibitor and inducer, respectively. Hepatocytes were treated with vehicle [0.1% dimethylsulfoxide, ritonavir (10 μM)], ketoconazole (10 μM), efavirenz (10 μM), or rifampin (10 μM) for 4 days. On day 5, erlotinib (5 μM) was incubated with the above agents for another 24-48 hours. Concentrations of erlotinib and O-desmethyl erlotinib were quantitated in collected samples (combined lysate and medium) using liquid chromatography and tandem mass spectrometry. The half-life (t(½)) of erlotinib increased from 10.6 ± 2.6 to 153 ± 80 and 23.9 ± 4.8 hours, respectively, upon treatment with ritonavir and ketoconazole. The apparent intrinsic clearance (C(Lint, app)) of erlotinib was lowered 16-fold by ritonavir and 1.9-fold by ketoconazole. Efavirenz and rifampin decreased t1/2 of erlotinib from 10.3 ± 1.1 to 5.0 ± 1.5 and 3.4 ± 0.2 hours, respectively. Efavirenz and rifampin increased the C(Lint, app) of erlotinib by 2.2- and 2-fold, respectively. Our results suggest that to achieve desired drug exposure, the clinically used dose (150 mg daily) of erlotinib may have to be significantly reduced (25 mg every other day) or increased (300 mg daily), respectively, when ritonavir or efavirenz is coadministered.
Figures



References
-
- Abbas R, Fettner S, Riek M, Bulsara S, Pantze M, Hamilton M, Frohna P, Rakhit A. (2003) A drug-drug interaction study to evaluate the effect of rifampicin on the pharmacokinetics of the EGF receptor tyrosine kinase inhibitor, erlotinib, in healthy subjects. Proc Am Soc Clin Oncol 22:548
-
- Backman JT, Granfors MT, Neuvonen PJ. (2006) Rifampicin is only a weak inducer of CYP1A2-mediated presystemic and systemic metabolism: studies with tizanidine and caffeine. Eur J Clin Pharmacol 62:451–461 - PubMed
-
- Beumer JH. (2013) Without therapeutic drug monitoring, there is no personalized cancer care. Clin Pharmacol Ther 93:228–230 - PubMed
-
- Boffito M, Acosta E, Burger D, Fletcher CV, Flexner C, Garaffo R, Gatti G, Kurowski M, Perno CF, Peytavin G, et al. (2005) Therapeutic drug monitoring and drug-drug interactions involving antiretroviral drugs. Antivir Ther 10:469–477 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

