Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis
- PMID: 23913274
- PMCID: PMC3867145
- DOI: 10.1038/nature12428
Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis
Abstract
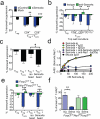
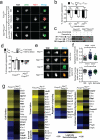
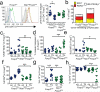
Regulatory T cells (Treg cells) have a crucial role in the immune system by preventing autoimmunity, limiting immunopathology, and maintaining immune homeostasis. However, they also represent a major barrier to effective anti-tumour immunity and sterilizing immunity to chronic viral infections. The transcription factor Foxp3 has a major role in the development and programming of Treg cells. The relative stability of Treg cells at inflammatory disease sites has been a highly contentious subject. There is considerable interest in identifying pathways that control the stability of Treg cells as many immune-mediated diseases are characterized by either exacerbated or limited Treg-cell function. Here we show that the immune-cell-expressed ligand semaphorin-4a (Sema4a) and the Treg-cell-expressed receptor neuropilin-1 (Nrp1) interact both in vitro, to potentiate Treg-cell function and survival, and in vivo, at inflammatory sites. Using mice with a Treg-cell-restricted deletion of Nrp1, we show that Nrp1 is dispensable for suppression of autoimmunity and maintenance of immune homeostasis, but is required by Treg cells to limit anti-tumour immune responses and to cure established inflammatory colitis. Sema4a ligation of Nrp1 restrained Akt phosphorylation cellularly and at the immunologic synapse by phosphatase and tensin homologue (PTEN), which increased nuclear localization of the transcription factor Foxo3a. The Nrp1-induced transcriptome promoted Treg-cell stability by enhancing quiescence and survival factors while inhibiting programs that promote differentiation. Importantly, this Nrp1-dependent molecular program is evident in intra-tumoral Treg cells. Our data support a model in which Treg-cell stability can be subverted in certain inflammatory sites, but is maintained by a Sema4a-Nrp1 axis, highlighting this pathway as a potential therapeutic target that could limit Treg-cell-mediated tumour-induced tolerance without inducing autoimmunity.
Figures

References
-
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. - PubMed
-
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. - PubMed
-
- Miyao T, et al. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012;36:262–275. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

