TraG encoded by the pIP501 type IV secretion system is a two-domain peptidoglycan-degrading enzyme essential for conjugative transfer
- PMID: 23913323
- PMCID: PMC3807477
- DOI: 10.1128/JB.02263-12
TraG encoded by the pIP501 type IV secretion system is a two-domain peptidoglycan-degrading enzyme essential for conjugative transfer
Abstract
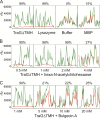
pIP501 is a conjugative broad-host-range plasmid frequently present in nosocomial Enterococcus faecalis and Enterococcus faecium isolates. We focus here on the functional analysis of the type IV secretion gene traG, which was found to be essential for pIP501 conjugative transfer between Gram-positive bacteria. The TraG protein, which localizes to the cell envelope of E. faecalis harboring pIP501, was expressed and purified without its N-terminal transmembrane helix (TraGΔTMH) and shown to possess peptidoglycan-degrading activity. TraGΔTMH was inhibited by specific lytic transglycosylase inhibitors hexa-N-acetylchitohexaose and bulgecin A. Analysis of the TraG sequence suggested the presence of two domains which both could contribute to the observed cell wall-degrading activity: an N-terminal soluble lytic transglycosylase domain (SLT) and a C-terminal cysteine-, histidine-dependent amidohydrolases/peptidases (CHAP) domain. The protein domains were expressed separately, and both degraded peptidoglycan. A change of the conserved glutamate residue in the putative catalytic center of the SLT domain (E87) to glycine resulted in almost complete inactivity, which is consistent with this part of TraG being a predicted lytic transglycosylase. Based on our findings, we propose that TraG locally opens the peptidoglycan to facilitate insertion of the Gram-positive bacterial type IV secretion machinery into the cell envelope.
Figures


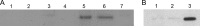

References
-
- Garcillán-Barcia MP, Francia MV, de la Cruz F. 2009. The diversity of conjugative relaxases and its application in plasmid classification. FEMS Microbiol. Rev. 33:657–687 - PubMed
-
- Porter CJ, Bantwal R, Bannam TL, Rosado CJ, Pearce MC, Adams V, Lyras D, Whisstock JC, Rood JI. 2012. The conjugation protein TcpC from Clostridium perfringens is structurally related to the type IV secretion system protein VirB8 from Gram-negative bacteria. Mol. Microbiol. 83:275–288 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

