Mitochondrial reactive oxygen species: which ROS signals cardioprotection?
- PMID: 23913710
- PMCID: PMC3798754
- DOI: 10.1152/ajpheart.00858.2012
Mitochondrial reactive oxygen species: which ROS signals cardioprotection?
Abstract
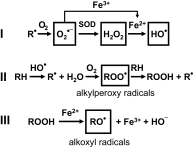
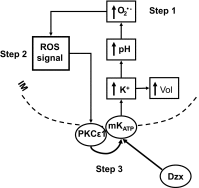
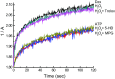
Mitochondria are the major effectors of cardioprotection by procedures that open the mitochondrial ATP-sensitive potassium channel (mitoKATP), including ischemic and pharmacological preconditioning. MitoKATP opening leads to increased reactive oxygen species (ROS), which then activate a mitoKATP-associated PKCε, which phosphorylates mitoKATP and leaves it in a persistent open state (Costa AD, Garlid KD. Am J Physiol Heart Circ Physiol 295, H874-H882, 2008). The ROS responsible for this effect is not known. The present study focuses on superoxide (O2(·-)), hydrogen peroxide (H2O2), and hydroxyl radical (HO(·)), each of which has been proposed as the signaling ROS. Feedback activation of mitoKATP provides an ideal setting for studying endogenous ROS signaling. Respiring rat heart mitochondria were preincubated with ATP and diazoxide, together with an agent being tested for interference with this process, either by scavenging ROS or by blocking ROS transformations. The mitochondria were then assayed to determine whether or not the persistent phosphorylated open state was achieved. Dimethylsulfoxide (DMSO), dimethylformamide (DMF), deferoxamine, Trolox, and bromoenol lactone each interfered with formation of the ROS-dependent open state. Catalase did not interfere with this step. We also found that DMF blocked cardioprotection by both ischemic preconditioning and diazoxide. The lack of a catalase effect and the inhibitory effects of agents acting downstream of HO(·) excludes H2O2 as the endogenous signaling ROS. Taken together, the results support the conclusion that the ROS message is carried by a downstream product of HO(·) and that it is probably a product of phospholipid oxidation.
Keywords: KATP channels; ROS signaling; cardiac ischemia; cardioprotection; mitochondria; reactive oxygen species.
Figures








Similar articles
-
Intramitochondrial signaling: interactions among mitoKATP, PKCepsilon, ROS, and MPT.Am J Physiol Heart Circ Physiol. 2008 Aug;295(2):H874-82. doi: 10.1152/ajpheart.01189.2007. Epub 2008 Jun 27. Am J Physiol Heart Circ Physiol. 2008. PMID: 18586884 Free PMC article.
-
Ischemic preconditioning requires increases in reactive oxygen release independent of mitochondrial K+ channel activity.Free Radic Biol Med. 2006 Feb 1;40(3):469-79. doi: 10.1016/j.freeradbiomed.2005.08.041. Epub 2005 Nov 9. Free Radic Biol Med. 2006. PMID: 16443162
-
GSK-3β at the crossroads in the signalling of heart preconditioning: implication of mTOR and Wnt pathways.Cardiovasc Res. 2011 Apr 1;90(1):49-56. doi: 10.1093/cvr/cvr002. Epub 2011 Jan 13. Cardiovasc Res. 2011. PMID: 21233250
-
Mitochondria "THE" target of myocardial conditioning.Am J Physiol Heart Circ Physiol. 2018 Nov 1;315(5):H1215-H1231. doi: 10.1152/ajpheart.00124.2018. Epub 2018 Jul 13. Am J Physiol Heart Circ Physiol. 2018. PMID: 30004243 Review.
-
The role of mitochondria in protection of the heart by preconditioning.Biochim Biophys Acta. 2007 Aug;1767(8):1007-31. doi: 10.1016/j.bbabio.2007.05.008. Epub 2007 Jun 2. Biochim Biophys Acta. 2007. PMID: 17631856 Free PMC article. Review.
Cited by
-
PRKCE gene encoding protein kinase C-epsilon-Dual roles at sarcomeres and mitochondria in cardiomyocytes.Gene. 2016 Sep 15;590(1):90-6. doi: 10.1016/j.gene.2016.06.016. Epub 2016 Jun 13. Gene. 2016. PMID: 27312950 Free PMC article. Review.
-
Cardioprotective mechanisms of mitochondria-targeted S-nitrosating agent and adenosine triphosphate-sensitive potassium channel opener are mutually exclusive.JTCVS Open. 2021 Aug 8;8:338-354. doi: 10.1016/j.xjon.2021.07.036. eCollection 2021 Dec. JTCVS Open. 2021. PMID: 36004142 Free PMC article.
-
Low dose radiation prevents doxorubicin-induced cardiotoxicity.Oncotarget. 2017 Dec 7;9(1):332-345. doi: 10.18632/oncotarget.23013. eCollection 2018 Jan 2. Oncotarget. 2017. PMID: 29416617 Free PMC article.
-
SARS-CoV-2 Mediated Endothelial Dysfunction: The Potential Role of Chronic Oxidative Stress.Front Physiol. 2021 Jan 15;11:605908. doi: 10.3389/fphys.2020.605908. eCollection 2020. Front Physiol. 2021. PMID: 33519510 Free PMC article. Review.
-
Mitochondrial Volume Regulation and Swelling Mechanisms in Cardiomyocytes.Antioxidants (Basel). 2023 Jul 28;12(8):1517. doi: 10.3390/antiox12081517. Antioxidants (Basel). 2023. PMID: 37627512 Free PMC article. Review.
References
-
- Andrukhiv A, Costa AD, West IC, Garlid KD. Opening mitoKATP increases superoxide generation from complex I of the electron transport chain. Am J Physiol Heart Circ Physiol 291: H2067–H2074, 2006 - PubMed
-
- Babbs CF, Steiner MG. Detection and quantitation of hydroxyl radical using dimethyl sulfoxide as molecular probe. Methods Enzymol 186: 137–147, 1990 - PubMed
-
- Baines CP, Goto M, Downey JM. Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. J Mol Cell Cardiol 29: 207–216, 1997 - PubMed
-
- Balafanova Z, Bolli R, Zhang J, Zheng Y, Pass JM, Bhatnagar A, Tang XL, Wang O, Cardwell E, Ping P. Nitric oxide (NO) induces nitration of protein kinase Cepsilon (PKCepsilon), facilitating PKCepsilon translocation via enhanced PKCepsilon-RACK2 interactions: a novel mechanism of no-triggered activation of PKCepsilon. J Biol Chem 277: 15021–15027, 2002 - PubMed
-
- Beavis AD, Brannan RD, Garlid KD. Swelling and contraction of the mitochondrial matrix. I. A structural interpretation of the relationship between light scattering and matrix volume. J Biol Chem 260: 13424–13433, 1985 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

