A force of nature: molecular mechanisms of mechanoperception in plants
- PMID: 23913953
- PMCID: PMC3817949
- DOI: 10.1093/jxb/ert204
A force of nature: molecular mechanisms of mechanoperception in plants
Abstract
The ability to sense and respond to a wide variety of mechanical stimuli-gravity, touch, osmotic pressure, or the resistance of the cell wall-is a critical feature of every plant cell, whether or not it is specialized for mechanotransduction. Mechanoperceptive events are an essential part of plant life, required for normal growth and development at the cell, tissue, and whole-plant level and for the proper response to an array of biotic and abiotic stresses. One current challenge for plant mechanobiologists is to link these physiological responses to specific mechanoreceptors and signal transduction pathways. Here, we describe recent progress in the identification and characterization of two classes of putative mechanoreceptors, ion channels and receptor-like kinases. We also discuss how the secondary messenger Ca(2+) operates at the centre of many of these mechanical signal transduction pathways.
Keywords: Calcium; cell-wall integrity; mechanoperception; mechanosensitive ion channels; receptor-like kinases; thigmomorphogenesis..
Figures
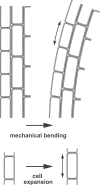


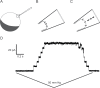
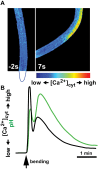
References
-
- Adamec L. 2012. Firing and resetting characteristics of carnivorous Utricularia reflexa traps: physiological or only physical regulation of trap triggering? Phyton-Annales Rei Botanicae 52, 281–290
-
- Anten NPR, Alcala-Herrera R, Schieving F, Onoda Y. 2010. Wind and mechanical stimuli differentially affect leaf traits in Plantago major . New Phytologist 188, 554–564 - PubMed
-
- Arnadottir J, Chalfie M. 2010. Eukaryotic mechanosensitive channels. Annual Review of Biophysics 39, 111–137 - PubMed
-
- Balleza D, Gomez-Lagunas F. 2009. Conserved motifs in mechanosensitive channels MscL and MscS. European Biophysical Journal 38, 1013–1027 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

