Influence of RNA structural elements on Ty1 retrotransposition
- PMID: 23914314
- PMCID: PMC3681743
- DOI: 10.4161/mge.25060
Influence of RNA structural elements on Ty1 retrotransposition
Abstract
The long-terminal repeat (LTR)-retrotransposon Ty1 is a mobile genetic element that replicates through an RNA intermediate. Retroelement genomic transcripts contain internal structures fundamental to gene expression and propagation. In addition, long non-coding antisense RNAs overlap the 5'-terminal region of the genomic RNA and confer post-translational copy number control. Although LTR- retrotransposons are functionally related to retroviruses, little is known about the structural determinants required for genomic RNA packaging or reverse transcription. This commentary summarizes two recent papers that provide the first snapshot of genomic RNA structures from the retrotransposon Ty1 involved in transposition. We combined structural approaches with functional and genetic assays to determine if antisense RNAs anneal with the genomic RNA. Analysis of various steps in the Ty1 life cycle showed that a novel RNA pseudoknot contributes to retrotransposon function. Comparing different RNA states provides additional information about regions potentially involved in Ty1 RNA dimerization or packaging.
Keywords: RNA packaging; RNA structure; Ty1 retrotransposon; pseudoknot; reverse transcription.
Figures
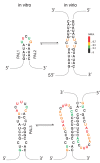

Comment on
- Purzycka KJ, Legiewicz M, Matsuda E, Eizentstat LD, Lusvarghi S, Saha A, et al. Exploring Ty1 retrotransposon RNA structure within virus-like particles. Nucleic Acids Res. 2013;41:463–73. doi: 10.1093/nar/gks983.
Similar articles
-
Characterizing the functions of Ty1 Gag and the Gag-derived restriction factor p22/p18.Mob Genet Elements. 2016 Mar 7;6(2):e1154637. doi: 10.1080/2159256X.2016.1154637. eCollection 2016 Mar-Apr. Mob Genet Elements. 2016. PMID: 27141325 Free PMC article.
-
Determinants of Genomic RNA Encapsidation in the Saccharomyces cerevisiae Long Terminal Repeat Retrotransposons Ty1 and Ty3.Viruses. 2016 Jul 14;8(7):193. doi: 10.3390/v8070193. Viruses. 2016. PMID: 27428991 Free PMC article. Review.
-
Exploring Ty1 retrotransposon RNA structure within virus-like particles.Nucleic Acids Res. 2013 Jan 7;41(1):463-73. doi: 10.1093/nar/gks983. Epub 2012 Oct 23. Nucleic Acids Res. 2013. PMID: 23093595 Free PMC article.
-
Retroviral-like determinants and functions required for dimerization of Ty1 retrotransposon RNA.RNA Biol. 2019 Dec;16(12):1749-1763. doi: 10.1080/15476286.2019.1657370. Epub 2019 Aug 30. RNA Biol. 2019. PMID: 31469343 Free PMC article.
-
The Ty1 LTR-Retrotransposon of Budding Yeast, Saccharomyces cerevisiae.Microbiol Spectr. 2015 Apr;3(2):MDNA3-0053-2014. doi: 10.1128/microbiolspec.MDNA3-0053-2014. Microbiol Spectr. 2015. PMID: 26104690 Review.
Cited by
-
Structure-Function Model for Kissing Loop Interactions That Initiate Dimerization of Ty1 RNA.Viruses. 2017 Apr 26;9(5):93. doi: 10.3390/v9050093. Viruses. 2017. PMID: 28445416 Free PMC article.
-
Saccharomyces cerevisiae RNA lariat debranching enzyme, Dbr1p, is required for completion of reverse transcription by the retrovirus-like element Ty1 and cleaves branched Ty1 RNAs.Mol Genet Genomics. 2021 Mar;296(2):409-422. doi: 10.1007/s00438-020-01753-y. Epub 2021 Jan 19. Mol Genet Genomics. 2021. PMID: 33464395
-
Characterizing the functions of Ty1 Gag and the Gag-derived restriction factor p22/p18.Mob Genet Elements. 2016 Mar 7;6(2):e1154637. doi: 10.1080/2159256X.2016.1154637. eCollection 2016 Mar-Apr. Mob Genet Elements. 2016. PMID: 27141325 Free PMC article.
-
Determinants of Genomic RNA Encapsidation in the Saccharomyces cerevisiae Long Terminal Repeat Retrotransposons Ty1 and Ty3.Viruses. 2016 Jul 14;8(7):193. doi: 10.3390/v8070193. Viruses. 2016. PMID: 27428991 Free PMC article. Review.
-
The Ty1 LTR-retrotransposon of budding yeast, Saccharomyces cerevisiae.Microbiol Spectr. 2015 Apr 1;3(2):1-35. doi: 10.1128/microbiolspec.MDNA3-0053-2014. Microbiol Spectr. 2015. PMID: 25893143 Free PMC article.
References
-
- Voytas DF, Boeke JD. (2002) In Craig, N. L., Craigie, R., Gellert, M. and Lambowitz, A. M. (eds.), Mobile DNA II ASM Press, Washington, DC, pp. 614-630
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
