Immobilization of Cell-Adhesive Laminin Peptides in Degradable PEGDA Hydrogels Influences Endothelial Cell Tubulogenesis
- PMID: 23914330
- PMCID: PMC3731677
- DOI: 10.1089/biores.2013.0021
Immobilization of Cell-Adhesive Laminin Peptides in Degradable PEGDA Hydrogels Influences Endothelial Cell Tubulogenesis
Abstract
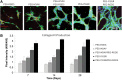
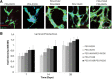
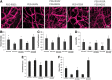
Attachment, spreading, and organization of endothelial cells into tubule networks are mediated by interactions between cells in the extracellular microenvironment. Laminins are key extracellular matrix components and regulators of cell adhesion, migration, and proliferation. In this study, laminin-derived peptides were conjugated to poly(ethylene glycol) (PEG) monoacrylate and covalently incorporated into degradable PEG diacrylate (PEGDA) hydrogels to investigate the influence of these peptides on endothelial cellular adhesion and function in organizing into tubule networks. Degradable PEGDA hydrogels were synthesized by incorporating a matrix metalloproteinase (MMP)-sensitive peptide, GGGPQGIWGQGK (abbreviated PQ), into the polymer backbone. The secretion of MMP-2 and MMP-9 by endothelial cells promotes polymer degradation and consequently cell migration. We demonstrate the formation of extensive networks of tubule-like structures by encapsulated human umbilical vein endothelial cells in hydrogels with immobilized synthetic peptides. The resulting structures were stabilized by pericyte precursor cells (10T1/2s) in vitro. During tubule formation and stabilization, extracellular matrix proteins such as collagen IV and laminin were deposited. Tubules formed in the matrix of metalloproteinase sensitive hydrogels were visualized from 7 days to 4 weeks in response to different combination of peptides. Moreover, hydrogels functionalized with laminin peptides and transplanted in a mouse cornea supported the ingrowth and attachment of endothelial cells to the hydrogel during angiogenesis. Results of this study illustrate the use of laminin-derived peptides as potential candidates for modification of biomaterials to support angiogenesis.
Keywords: angiogenesis; biomaterials; extracellular matrix; peptides; tissue engineering.
Figures
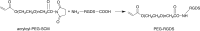

References
-
- Atala A. Bauer SB. Soker S. Yoo JJ. Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–1246. - PubMed
-
- Jain RK. Au P. Tam J. Duda DG. Fukumura D. Engineering vascularized tissue. Nat Biotechnol. 2005;23:821–823. - PubMed
-
- Francis ME. Uriel S. Brey EM. Endothelial cell-matrix interactions in neovascularization. Tissue Eng Part B Rev. 2008;14:19–32. - PubMed
-
- Miner JH. Laminins and their roles in mammals. Microsc Res Tech. 2008;71:349–356. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
