10-year trend in quantity and quality of pediatric randomized controlled trials published in mainland China: 2002-2011
- PMID: 23914882
- PMCID: PMC3750923
- DOI: 10.1186/1471-2431-13-113
10-year trend in quantity and quality of pediatric randomized controlled trials published in mainland China: 2002-2011
Abstract
Background: Quality assessment of pediatric randomized controlled trials (RCTs) in China is limited. The aim of this study was to evaluate the quantitative trends and quality indicators of RCTs published in mainland China over a recent 10-year period.
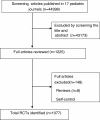
Methods: We individually searched all 17 available pediatric journals published in China from January 1, 2002 to December 30, 2011 to identify RCTs of drug treatment in participants under the age of 18 years. The quality was evaluated according to the Cochrane quality assessment protocol.
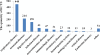
Results: Of 1287 journal issues containing 44398 articles, a total of 2.4% (1077/44398) articles were included in the analysis. The proportion of RCTs increased from 0.28% in 2002 to 0.32% in 2011. Individual sample sizes ranged from 10 to 905 participants (median 81 participants); 2.3% of the RCTs were multiple center trials; 63.9% evaluated Western medicine, 32.5% evaluated traditional Chinese medicine; 15% used an adequate method of random sequence generation; and 10.4% used a quasi-random method for randomization. Only 1% of the RCTs reported adequate allocation concealment and 0.6% reported the method of blinding. The follow-up period was from 7 days to 96 months, with a median of 7.5 months. There was incomplete outcome data reported in 8.3%, of which 4.5% (4/89) used intention-to-treat analysis. Only 0.4% of the included trials used adequate random sequence allocation, concealment and blinding. The articles published from 2007 to 2011 revealed an improvement in the randomization method compared with articles published from 2002 to 2006 (from 2.7% to 23.6%, p = 0.000).
Conclusions: In mainland China, the quantity of RCTs did not increase in the pediatric population, and the general quality was relatively poor. Quality improvements were suboptimal in the later 5 years.
Figures
Similar articles
-
Quality assessment of paediatric randomized controlled trials published in China from 1999 to 2022: a cross-sectional study.BMC Pediatr. 2024 May 27;24(1):364. doi: 10.1186/s12887-024-04839-3. BMC Pediatr. 2024. PMID: 38802810 Free PMC article.
-
Methodological reporting quality of randomized controlled trials: A survey of seven core journals of orthopaedics from Mainland China over 5 years following the CONSORT statement.Orthop Traumatol Surg Res. 2016 Nov;102(7):933-938. doi: 10.1016/j.otsr.2016.05.018. Epub 2016 Aug 8. Orthop Traumatol Surg Res. 2016. PMID: 27514437
-
Quality assessment of reporting of randomization, allocation concealment, and blinding in traditional Chinese medicine RCTs: a review of 3159 RCTs identified from 260 systematic reviews.Trials. 2011 May 13;12:122. doi: 10.1186/1745-6215-12-122. Trials. 2011. PMID: 21569452 Free PMC article. Review.
-
The quality of reporting of randomized controlled trials of traditional Chinese medicine: a survey of 13 randomly selected journals from mainland China.Clin Ther. 2007 Jul;29(7):1456-67. doi: 10.1016/j.clinthera.2007.07.023. Clin Ther. 2007. PMID: 17825697 Review.
-
Assessment of methodological quality and outcome measures of acute stroke randomized controlled trials in China in recent 15 years.J Evid Based Med. 2012 Aug;5(3):174-82. doi: 10.1111/j.1756-5391.2012.01190.x. J Evid Based Med. 2012. PMID: 23672224 Clinical Trial.
Cited by
-
Quality assessment of paediatric randomized controlled trials published in China from 1999 to 2022: a cross-sectional study.BMC Pediatr. 2024 May 27;24(1):364. doi: 10.1186/s12887-024-04839-3. BMC Pediatr. 2024. PMID: 38802810 Free PMC article.
-
Trends in the number and the quality of trial protocols involving children submitted to a French Institutional Review Board.BMC Med Res Methodol. 2017 Aug 23;17(1):130. doi: 10.1186/s12874-017-0395-4. BMC Med Res Methodol. 2017. PMID: 28835231 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical