Protecting group-free synthesis of 1,2-azaborines: a simple approach to the construction of BN-benzenoids
- PMID: 23914914
- PMCID: PMC3809131
- DOI: 10.1021/ja4073436
Protecting group-free synthesis of 1,2-azaborines: a simple approach to the construction of BN-benzenoids
Abstract
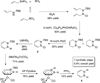
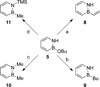
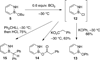
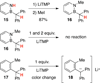
The protecting group-free synthesis of a versatile 1,2-azaborine synthon 5 is described. Previously inaccessible 1,2-azaborine derivatives, including the BN isostere of phenyl phenylacetate and BN1 triphenylmethane were prepared from 5 and characterized. The structural investigation of BN phenyl phenylacetate revealed the presence of a unique NH-carbonyl hydrogen bond that is not present in the corresponding carbonaceous analogue. The methyne CH in BN triphenylmethane was found to be less acidic than the corresponding proton in triphenylmethane. The gram-quantity synthesis of the parent 1,2-azaborine 4 was demonstrated, which enabled the characterization of its boiling point, density, refractive index, and its polarity on the ET(30) scale.
Figures





References
-
-
For an overview, see:. Campbell PG, Marwitz AJV, Liu S-Y. Angew. Chem. Int. Ed. 2012;51:6074–6092. Bosdet MJD, Piers WE. Can. J. Chem. 2009;87:8–29. Liu Z, Marder TB. Angew. Chem. Int. Ed. 2008;47:242–244. Abbey ER, Liu S-Y. Org. Biomol. Chem. 2013;11:2060–2069. Fritsch AJ. Chem. Heterocycl. Compd. 1977;30:381–440.
-
-
-
For leading references, see: Knack DH, Marshall JL, Harlow GP, Dudzik A, Szaleniec M, Liu S-Y, Heider J. Angew. Chem. Int. Ed. 2013;52:2599–2601. Marwitz AJV, Lamm AN, Zakharov LN, Vasiliu M, Dixon DA, Liu S-Y. Chem. Sci. 2012;3:825. Abbey ER, Zakharov LN, Liu S-Y. J. Am. Chem. Soc. 2010;132:16340–16342. Liu L, Marwitz AJ, Matthews BW, Liu S-Y. Angew. Chem. Int. Ed. 2009;48:6817–6819.
-
-
- Kranz M, Clark T. J. Org. Chem. 1992;57:5492–5500.
- Silva PJ, Ramos MJ. J. Org. Chem. 2009;74:6120–6129. - PubMed
- Fazen PJ, Burke LA. Inorg. Chem. 2006;45:2494–2500. - PubMed
- Matus MH, Liu S-Y, Dixon DA. J. Phys. Chem. A. 2010;114:2644–2654. - PubMed
- Abbey ER, Zakharov LN, Liu S-Y. J. Am. Chem. Soc. 2008;130:7250–7252. - PubMed
- Campbell PG, Abbey ER, Neiner D, Grant DJ, Dixon DA, Liu S-Y. J. Am. Chem. Soc. 2010;132:18048–18050. - PubMed
- Pan J, Kampf JW, Ashe AJ. Org. Lett. 2007;9:679–681. - PubMed
- Chrostowska A, Xu S, Lamm AN, Mazière A, Weber CD, Dargelos A, Baylère P, Graciaa A, Liu S-Y. J. Am. Chem. Soc. 2012;134:10279–10285. - PMC - PubMed
-
- Dewar MJS, Marr PA. J. Am. Chem. Soc. 1962;84:3782.
- Culling GC, Dewar MJS, Marr PA. J. Am. Chem. Soc. 1964;86:1125–1127.
- Davies KM, Dewar MJS, Rona P. J. Am. Chem. Soc. 1967;89:6294–6297.
-
- Ashe AJ, III, Fang X, Fang X, Kampf JW. Organometallics. 2001;20:5413–5418.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

