Interferon beta 1b following natalizumab discontinuation: one year, randomized, prospective, pilot trial
- PMID: 23915113
- PMCID: PMC3750382
- DOI: 10.1186/1471-2377-13-101
Interferon beta 1b following natalizumab discontinuation: one year, randomized, prospective, pilot trial
Abstract
Background: Natalizumab (NTZ) discontinuation leads to multiple sclerosis reactivation.The objective of this study is to compare disease activity in MS patients who continued on NTZ treatment to those who were switched to subcutaneous interferon 1b (IFNB) treatment.
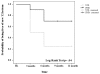
Methods: 1-year randomized, rater-blinded, parallel-group, pilot study (ClinicalTrial.gov ID: NCT01144052). Relapsing remitting MS patients on NTZ for ≥12 months who had been free of disease activity on this therapy (no relapses and disability progression for ≥6 months, no gadolinium-enhancing lesions on baseline MRI) were randomized to NTZ or IFNB. Primary endpoint was time to first on-study relapse. Additional clinical, MRI and safety parameters were assessed. Analysis was based on intention to treat.
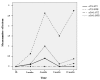
Results: 19 patients (NTZ n=10; IFNB n=9) with similar baseline characteristics were included. 78% of IFNB treated patients remained relapse free (NTZ group: 100%), and 25% remained free of new T2 lesions (NTZ group: 62.5%). While time to first on-study relapse was not significantly different between groups (p=0.125), many secondary clinical and radiological endpoints (number of relapses, proportion of relapse free patients, number of new T2 lesions) showed a trend, or were significant (new T2 lesions at month 6) in favoring NTZ.
Conclusions: De-escalation therapy from NTZ to IFNB over 1 year was associated with some clinical and radiological disease recurrence. Overall no major safety concerns were observed.
Figures
References
-
- Cada DJ, Levien T, Baker DE. Natalizumab. Hosp Pharm. 2005;40:336–346.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

