Hemoglobin induces inflammation after preterm intraventricular hemorrhage by methemoglobin formation
- PMID: 23915174
- PMCID: PMC3750409
- DOI: 10.1186/1742-2094-10-100
Hemoglobin induces inflammation after preterm intraventricular hemorrhage by methemoglobin formation
Abstract
Background: Cerebral intraventricular hemorrhage (IVH) is a major cause of severe neurodevelopmental impairment in preterm infants. To date, no therapy is available that prevents infants from developing serious neurological disability following IVH. Thus, to develop treatment strategies for IVH, it is essential to characterize the initial sequence of molecular events that leads to brain damage. In this study, we investigated extracellular hemoglobin (Hb) as a causal initiator of inflammation in preterm IVH.
Methods: Using a preterm rabbit pup model, we investigated the molecular mechanisms and events following IVH. We also characterized the concentrations of cell-free Hb metabolites and pro-inflammatory mediators in the cerebrospinal fluid (CSF) of preterm human infants and rabbit pups. Finally, Hb metabolites were evaluated as causal initiators of inflammation in primary rabbit astrocyte cell cultures.
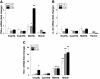
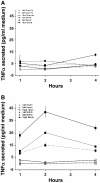
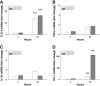
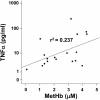
Results: Following IVH in preterm rabbit pups, the intraventricular CSF concentration of cell-free methemoglobin (metHb) increased from 24 to 72 hours and was strongly correlated with the concentration of TNFα at 72 hours (r2 = 0.896, P <0.001). Also, the mRNA expression of TNFα, IL-1β, and Toll-like receptor-4 and TNFα protein levels were significantly increased in periventricular tissue at 72 hours, which was accompanied by extensive astrocyte activation (that is, glial fibrillary acidic protein (GFAP)staining). Furthermore, exposure of primary rabbit astrocyte cell cultures to metHb caused a dose-dependent increase in TNFα mRNA and protein levels, which was not observed following exposure to oxyhemoglobin (oxyHb) or hemin. Finally, a positive correlation (r2 = 0.237, P <0.03) between metHb and TNFα concentrations was observed in the CSF of preterm human infants following IVH.
Conclusions: Following preterm IVH, increased metHb formation in the intraventricular space induces expression of pro-inflammatory cytokines. Thus, the formation of metHb might be a crucial initial event in the development of brain damage following preterm IVH. Accordingly, removal, scavenging, or neutralization of Hb could present a therapeutic opportunity and plausible approach to decreasing the damage in the immature brain following preterm IVH.
Figures





References
-
- Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB, Finer NN, Ehrenkranz RA, Duara S, Sánchez PJ, O'Shea TM, Goldberg RN, Van Meurs KP, Faix RG, Phelps DL, Frantz ID 3rd, Watterberg KL, Saha S, Das A, Higgins RD. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

