Risk of hyperkalemia in patients with moderate chronic kidney disease initiating angiotensin converting enzyme inhibitors or angiotensin receptor blockers: a randomized study
- PMID: 23915518
- PMCID: PMC3750227
- DOI: 10.1186/1756-0500-6-306
Risk of hyperkalemia in patients with moderate chronic kidney disease initiating angiotensin converting enzyme inhibitors or angiotensin receptor blockers: a randomized study
Abstract
Background: Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers are renoprotective but both may increase serum potassium concentrations in patients with chronic kidney disease (CKD). The proportion of affected patients, the optimum follow-up period and whether there are differences between drugs in the development of this complication remain to be ascertained.
Methods: In a randomized, double-blind, phase IV, controlled, crossover study we recruited 30 patients with stage 3 CKD under restrictive eligibility criteria and strict dietary control. With the exception of withdrawals, each patient was treated with olmesartan and enalapril separately for 3 months each, with a 1-week wash-out period between treatments. Patients were clinically assessed on 10 occasions via measurements of serum and urine samples. We used the Cochran-Mantel-Haenszel statistics for comparison of categorical data between groups. Comparisons were also made using independent two-sample t-tests and Welch's t-test. Analysis of variance (ANOVA) was performed when necessary. We used either a Mann-Whitney or Kruskal-Wallis test if the distribution was not normal or the variance not homogeneous.
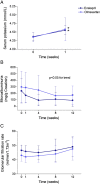
Results: Enalapril and olmesartan increased serum potassium levels similarly (0.3 mmol/L and 0.24 mmol/L respectively). The percentage of patients presenting hyperkalemia higher than 5 mmol/L did not differ between treatments: 37% for olmesartan and 40% for enalapril. The mean e-GFR ranged 46.3 to 48.59 ml/mint/1.73 m2 in those treated with olmesartan and 46.8 to 48.3 ml/mint/1.73 m2 in those with enalapril and remained unchanged at the end of the study. The decreases in microalbuminuria were also similar (23% in olmesartan and 29% in enalapril patients) in the 4 weeks time point. The percentage of patients presenting hyperkalemia, even after a two month period, did not differ between treatments. There were no appreciable changes in sodium and potassium urinary excretion.
Conclusions: Disturbances in potassium balance upon treatment with either olmesartan or enalapril are frequent and without differences between groups. The follow-up of these patients should include control of potassium levels, at least after the first week and the first and second month after initiating treatment.
Trial registration: The trial EudraCT "2008-002191-98".
Figures

References
-
- Ponce SP, Jennings AE, Madias NE, Harrington JT. Drug-induced hyperkalemia. Med (Baltimore) 1985;64:357–370. - PubMed
-
- Mogensen CE, Neldam S, Tikkanen I, Oren S, Viskoper R, Watts RW, Cooper ME. Randomised controlled trial of dual blockade of renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes. BMJ. 2000;321:1440–1444. doi: 10.1136/bmj.321.7274.1440. - DOI - PMC - PubMed
-
- De Denus S, Tardif JC, White M, Bourassa MG, Racine N, Levesque S, Ducharme A. Quantification of the risk and predictors of hyperkalemia in patients with left ventricular dysfunction: a retrospective analysis of the studies of left ventricular dysfunction (SOLVD) trials. Am Heart J. 2006;152:705–712. doi: 10.1016/j.ahj.2006.05.030. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

