Storing maternal memories: hypothesizing an interaction of experience and estrogen on sensory cortical plasticity to learn infant cues
- PMID: 23916405
- PMCID: PMC3788048
- DOI: 10.1016/j.yfrne.2013.07.008
Storing maternal memories: hypothesizing an interaction of experience and estrogen on sensory cortical plasticity to learn infant cues
Abstract
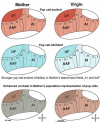
Much of the literature on maternal behavior has focused on the role of infant experience and hormones in a canonical subcortical circuit for maternal motivation and maternal memory. Although early studies demonstrated that the cerebral cortex also plays a significant role in maternal behaviors, little has been done to explore what that role may be. Recent work though has provided evidence that the cortex, particularly sensory cortices, contains correlates of sensory memories of infant cues, consistent with classical studies of experience-dependent sensory cortical plasticity in non-maternal paradigms. By reviewing the literature from both the maternal behavior and sensory cortical plasticity fields, focusing on the auditory modality, we hypothesize that maternal hormones (predominantly estrogen) may act to prime auditory cortical neurons for a longer-lasting neural trace of infant vocal cues, thereby facilitating recognition and discrimination. This couldthen more efficiently activate the subcortical circuit to elicit and sustain maternal behavior.
Keywords: Auditory cortex; Estradiol; Estrogen; Experience-dependent plasticity; Infant; Maternal behavior; Memory; Natural stimulus; Parenting; Vocalization.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures



References
-
- Adkins-Regan E. Hormonal organization and activation: Evolutionary implications and questions. General and Comparative Endocrinology. 2012;176:279–285. - PubMed
-
- Afonso VM, Grella SL, Chatterjee D, Fleming AS. Previous maternal experience affects accumbal dopaminergic responses to pup-stimuli. Brain Research. 2008;1198:115–123. - PubMed
-
- Bakin JS, Weinberger NM. Classical conditioning induces cs-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Research. 1990;536:271–286. - PubMed
-
- Bao S, Chan VT, Merzenich MM. Cortical remodeling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

