Characterization of the phytochelatin synthase from the human parasitic nematode Ancylostoma ceylanicum
- PMID: 23916800
- PMCID: PMC3823645
- DOI: 10.1016/j.molbiopara.2013.07.003
Characterization of the phytochelatin synthase from the human parasitic nematode Ancylostoma ceylanicum
Abstract
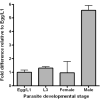
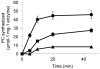
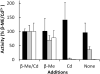
Hookworm disease is a debilitating worm infection that affects hundreds of millions of people. Despite the existence of anthelmintic drugs, reports have testified of a decrease in efficacy of these drugs. Therefore, it is imperative to find new drugs and drug targets for hookworm disease treatment. In this study we identify the gene encoding the phytochelatin synthase in the human hookworm, Ancylostoma ceylanicum (AcePCS). Phytochelatin synthase catalyzes the production of metal chelating peptides, the phytochelatins, from glutathione (GSH). In plants, algae, and fungi phytochelatin production is important for metal tolerance and detoxification. Phytochelatin synthase proteins also function in the elimination of xenobiotics by processing GSH S-conjugates. We found that in vitro AcePCS could both synthesize phytochelatins and hydrolyze a GSH S-conjugate. Interestingly, the enzyme works through a thiol-dependent and, notably, metal-independent mechanism for both transpeptidase (phytochelatin synthesis) and peptidase (hydrolysis of GSH S-conjugates) activities. AcePCS mRNAs are expressed in vivo throughout the life cycle of A. ceylanicum. Mature adult male hookworms isolated from the small intestines of their hosts displayed significantly enhanced expression of AcePCS with transcript levels 5-fold greater than other developmental forms. Although the role of AcePCS in A. ceylanicum biology has yet to be fully investigated the results reported here provide encouraging evidence of the potential that this enzyme holds as a target for new chemotherapeutic intervention.
Keywords: 2-mercaptoethanol; AcePCS; Ancylostoma ceylanicum phytochelatin synthase; EST; GSH; Glutathione; Hookworm; Metal toxicity; NTD; Neglected tropical disease; PCS; Phytochelatin; STNs; Xenobiotic metabolism; expressed sequence tag; glutathione; neglected tropical disease; phytochelatin synthase; qPCR; real-time quantitative PCR; soil-transmitted nematodes; β-ME.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures

References
-
- Brautigam A, Schaumloffel D, Preud’homme H, Thondorf I, Wesenberg D. Physiological characterization of cadmium-exposed Chlamydomonas reinhardtii. Plant Cell Environ. 2011;34:2071–82. - PubMed
-
- Brulle F, Cocquerelle C, Wamalah AN, Morgan AJ, Kille P, Lepretre A, et al. cDNA cloning and expression analysis of Eisenia fetida (Annelida: Oligochaeta) phytochelatin synthase under cadmium exposure. Ecotoxicol Environ Saf. 2008;71:47–55. - PubMed
-
- Clemens S, Schroeder JI, Degenkolb T. Caenorhabditis elegans expresses a functional phytochelatin synthase. Eur J Biochem. 2001;268:3640–3. - PubMed
-
- Cobbett C, Goldsbrough P. Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol. 2002;53:159–82. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

