Atherosclerosis and interferon-γ: new insights and therapeutic targets
- PMID: 23916809
- PMCID: PMC3844070
- DOI: 10.1016/j.tcm.2013.06.003
Atherosclerosis and interferon-γ: new insights and therapeutic targets
Abstract
Atherosclerosis is considered to be a chronic inflammatory disease of the arterial wall. Atherogenesis is accompanied by local production and release of inflammatory mediators, for which the macrophage is a major source. The proinflammatory cytokine, interferon (IFN)-γ derived from T cells, is expressed at high levels in atherosclerotic lesions. IFN-γ is the classic macrophage-activating factor, vital for both innate and adaptive immunity. It primes macrophages to produce chemokines and cytotoxic molecules and induces expression of genes that regulate lipid uptake. IFN-γ is a key trigger for the formation and release of reactive oxygen species. IFN-γ has important effects on endothelial cells, promoting expression of adhesion molecules. Atherogenic effects of IFN-γ have been shown in murine models where exogenous administration enhances atherosclerotic lesion formation while knockout of IFN-γ or its receptor reduces lesion size. IFN-γ signaling is largely mediated by a Janus kinase (JAK) to signal transduction and activator of transcription (STAT)1 cytosolic factor pathway. A clear understanding of IFN-γ effects on atherogenesis should enable development of novel targeted interventions for clinical use in the prevention and treatment of atherosclerosis. This review will discuss the actions of the cytokine IFN-γ and its complex effects on cells involved in atherosclerosis.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

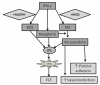

 , induces (red arrows) or inhibits (blue arrows) the expression of numerous genes in endothelial cells
, induces (red arrows) or inhibits (blue arrows) the expression of numerous genes in endothelial cells  , macrophages
, macrophages  , and SMC
, and SMC  . IFN-γ provokes monocyte/lymphocyte recruitment and infiltration into the subendothelium by induction of MCP-1, M-CSF, and release of adhesion molecules such as ICAM-1, VCAM-1, and P-selectin. IFN-γ promotes foam cell formation by increasing the uptake of modified LDL (mLDL
. IFN-γ provokes monocyte/lymphocyte recruitment and infiltration into the subendothelium by induction of MCP-1, M-CSF, and release of adhesion molecules such as ICAM-1, VCAM-1, and P-selectin. IFN-γ promotes foam cell formation by increasing the uptake of modified LDL (mLDL  ) by scavenger receptors, like CD36, SRA1, and CXCL16, and reducing cholesterol efflux (ABCA1). IFN-γ-induced release of MMPs enables SMC to migrate through tissue by degrading extracellular matrix. Mature SMC retain remarkable plasticity, become macrophage-like and contribute to the foam cell population of the intima. MMPs are proteolytic enzymes that can weaken and disrupt plaque structure, including the fibrous cap.
) by scavenger receptors, like CD36, SRA1, and CXCL16, and reducing cholesterol efflux (ABCA1). IFN-γ-induced release of MMPs enables SMC to migrate through tissue by degrading extracellular matrix. Mature SMC retain remarkable plasticity, become macrophage-like and contribute to the foam cell population of the intima. MMPs are proteolytic enzymes that can weaken and disrupt plaque structure, including the fibrous cap.References
-
- Amento EP, Ehsani N, Palmer H, Libby P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arteriosclerosis Thrombosis and Vascular Biology. 1991;11:1223–30. - PubMed
-
- Billiau A, Matthys P. Interferon-gamma: a historical perspective. Cytokine and Growth Factor Reviews. 2009;20(2):97–113. - PubMed
-
- Businaro R, Tagliani A, Buttari B, Profumo E, Ippoliti F, Di Cristofano C, et al. Cellular and molecular players in the atherosclerotic plaque progression. Annals of the New York Academy of Sciences. 2012;1262:134–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

