Relationship of lipoproteins to cardiovascular events: the AIM-HIGH Trial (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides and Impact on Global Health Outcomes)
- PMID: 23916935
- PMCID: PMC3862446
- DOI: 10.1016/j.jacc.2013.07.023
Relationship of lipoproteins to cardiovascular events: the AIM-HIGH Trial (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides and Impact on Global Health Outcomes)
Abstract
Objectives: This study sought to examine the relationship between niacin treatment, lipoproteins, and cardiovascular (CV) outcomes in this secondary analysis of the AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides and Impact on Global Health Outcomes) trial.
Background: During a 3-year follow-up in 3,414 patients with established CV disease and low high-density lipoprotein cholesterol (HDL-C) levels, combined niacin + low-density lipoprotein cholesterol (LDL-C)-lowering therapy did not reduce CV events compared with LDL-C-lowering therapy alone.
Methods: Subjects taking simvastatin and/or ezetimibe were randomized to receive extended-release (ER) niacin 1,500 to 2,000 mg or minimal immediate-release niacin (≤ 150 mg) as placebo at bedtime. LDL-C levels in both groups were maintained from 40 to 80 mg/dl. Hazard ratios were estimated by using Cox proportional hazards models for relationships between lipoproteins and the composite endpoint of CV death, myocardial infarction, acute coronary syndrome, ischemic stroke, or symptom-driven revascularization.
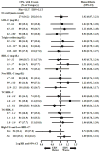
Results: CV outcomes were not associated with ER niacin in any baseline lipoprotein tertile. In a subset of patients in both the highest triglyceride (≥ 198 mg/dl) and lowest HDL-C (<33 mg/dl) tertiles, ER niacin showed a trend toward benefit (hazard ratio: 0.74, p = 0.073). In-trial LDL-C levels, non-HDL-C levels, and the total cholesterol/HDL-C ratio were positively associated with CV events in the control group, but these relationships were absent in the ER niacin group.
Conclusions: Baseline lipoprotein tertiles did not predict differential benefit or harm with ER niacin added to LDL-C-lowering therapy, but a small dyslipidemic subgroup may benefit. ER niacin attenuated expected relationships of lipoprotein risk factors with CV events, raising the possibility that nonlipoprotein actions of niacin could affect risk. (Niacin Plus Statin to Prevent Vascular Events [AIM-HIGH]; NCT00120289).
Keywords: CV; ER; G-protein–coupled receptor 109A; GPR109A; HDL-C; HR; LDL-C; TG; cardiovascular; cardiovascular events; clinical trial; extended-release; hazard ratio; high-density lipoprotein cholesterol; lipoproteins; low-density lipoprotein cholesterol; niacin; triglyceride.
Copyright © 2013 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Aim-High Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. New Engl J Med. 2011;365:2255–67. - PubMed
-
- Armitage J. HPS2-THRIVE: Randomized placebo-controlled trial of ER niacin and laropiprant in 25,673 patients with pre-existing cardiovascular disease. American College of Cardiology Annual Scientific Sessions; San Francisco: 2013.
-
- Lee JM, Robson MD, Yu LM, et al. Effects of high-dose modified-release nicotinic acid on atherosclerosis and vascular function: a randomized, placebo-controlled, magnetic resonance imaging study. J Am Coll Cardiol. 2009;54:1787–94. - PubMed
-
- Taylor AJ, Villines TC, Stanek EJ, et al. Extended-release niacin or ezetimibe and carotid intima-media thickness. New Engl J Med. 2009;361:2113–2122. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

