Two novel tyrosinase inhibitory sesquiterpenes induced by CuCl2 from a marine-derived fungus Pestalotiopsis sp. Z233
- PMID: 23917067
- PMCID: PMC3766860
- DOI: 10.3390/md11082713
Two novel tyrosinase inhibitory sesquiterpenes induced by CuCl2 from a marine-derived fungus Pestalotiopsis sp. Z233
Abstract
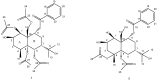
Two new sesquiterpenes, 1β,5α,6α,14-tetraacetoxy-9α-benzoyloxy-7β H-eudesman-2β,11-diol (1) and 4α,5α-diacetoxy-9α-benzoyloxy-7βH-eudesman-1β,2β,11, 14-tetraol (2), were produced as stress metabolites in the cultured mycelia of Pestalotiopsis sp. Z233 isolated from the algae Sargassum horneri in response to abiotic stress elicitation by CuCl2. Their structures were established by spectroscopic means. New compounds 1 and 2 showed tyrosinase inhibitory activities with IC50 value of 14.8 µM and 22.3 µM.
Figures
References
-
- Yamazaki H., Rotinsulu H., Kaneko T., Murakami K., Fujiwara H., Ukai K., Namikoshi M. A new dibenz[b,e]oxepine derivative, 1-hydroxy-10-methoxy-dibenz[b,e]oxepin-6,11-dione, from a marine-derived fungus, Beauveria bassiana TPU942. Mar. Drugs. 2012;10:2691–2697. doi: 10.3390/md10122691. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources