Out of balance--systemic iron homeostasis in iron-related disorders
- PMID: 23917168
- PMCID: PMC3775241
- DOI: 10.3390/nu5083034
Out of balance--systemic iron homeostasis in iron-related disorders
Abstract
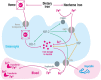
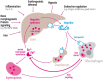
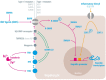
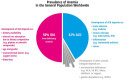
Iron is an essential element in our daily diet. Most iron is required for the de novo synthesis of red blood cells, where it plays a critical role in oxygen binding to hemoglobin. Thus, iron deficiency causes anemia, a major public health burden worldwide. On the other extreme, iron accumulation in critical organs such as liver, heart, and pancreas causes organ dysfunction due to the generation of oxidative stress. Therefore, systemic iron levels must be tightly balanced. Here we focus on the regulatory role of the hepcidin/ferroportin circuitry as the major regulator of systemic iron homeostasis. We discuss how regulatory cues (e.g., iron, inflammation, or hypoxia) affect the hepcidin response and how impairment of the hepcidin/ferroportin regulatory system causes disorders of iron metabolism.
Figures

References
-
- Domke A., Großklaus R., Niemann B., Przyrembel H., Richter K., Schmidt E., Weißenborn A., Wörner B., Ziegenhagen R. In: Utilisation of Minerals in Nutrients—Toxicologic and Nutrition-Physiologic Aspects. Wissenschaft B., editor. BfR; Halem, Germany: 2004. p. 323.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

