Prelamin A causes progeria through cell-extrinsic mechanisms and prevents cancer invasion
- PMID: 23917225
- PMCID: PMC3758871
- DOI: 10.1038/ncomms3268
Prelamin A causes progeria through cell-extrinsic mechanisms and prevents cancer invasion
Abstract
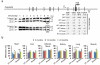
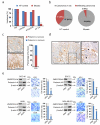
Defining the relationship between ageing and cancer is a crucial but challenging task. Mice deficient in Zmpste24, a metalloproteinase mutated in human progeria and involved in nuclear prelamin A maturation, recapitulate multiple features of ageing. However, their short lifespan and serious cell-intrinsic and cell-extrinsic alterations restrict the application and interpretation of carcinogenesis protocols. Here we present Zmpste24 mosaic mice that lack these limitations. Zmpste24 mosaic mice develop normally and keep similar proportions of Zmpste24-deficient (prelamin A-accumulating) and Zmpste24-proficient (mature lamin A-containing) cells throughout life, revealing that cell-extrinsic mechanisms are preeminent for progeria development. Moreover, prelamin A accumulation does not impair tumour initiation and growth, but it decreases the incidence of infiltrating oral carcinomas. Accordingly, silencing of ZMPSTE24 reduces human cancer cell invasiveness. Our results support the potential of cell-based and systemic therapies for progeria and highlight ZMPSTE24 as a new anticancer target.
Figures



References
-
- Burtner CR, Kennedy BK. Progeria syndromes and ageing: what is the connection? Nat. Rev. Mol. Cell. Biol. 2010;11:567–578. - PubMed
-
- Pendás AM, et al. Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nat. Genet. 2002;31:94–99. - PubMed
-
- De Sandre-Giovannoli A, et al. Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003;300:2055. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

