Auditory ganglion source of Sonic hedgehog regulates timing of cell cycle exit and differentiation of mammalian cochlear hair cells
- PMID: 23918393
- PMCID: PMC3752254
- DOI: 10.1073/pnas.1222341110
Auditory ganglion source of Sonic hedgehog regulates timing of cell cycle exit and differentiation of mammalian cochlear hair cells
Abstract
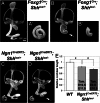
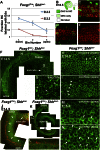
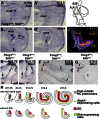
Neural precursor cells of the central nervous system undergo successive temporal waves of terminal division, each of which is soon followed by the onset of cell differentiation. The organ of Corti in the mammalian cochlea develops differently, such that precursors at the apex are the first to exit from the cell cycle but the last to begin differentiating as mechanosensory hair cells. Using a tissue-specific knockout approach in mice, we show that this unique temporal pattern of sensory cell development requires that the adjacent auditory (spiral) ganglion serve as a source of the signaling molecule Sonic hedgehog (Shh). In the absence of this signaling, the cochlear duct is shortened, sensory hair cell precursors exit from the cell cycle prematurely, and hair cell differentiation closely follows cell cycle exit in a similar apical-to-basal direction. The dynamic relationship between the restriction of Shh expression in the developing spiral ganglion and its proximity to regions of the growing cochlear duct dictates the timing of terminal mitosis of hair cell precursors and their subsequent differentiation.
Keywords: Atoh1; morphogenesis; tonotopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Corwin JT, Warchol ME. Auditory hair cells: Structure, function, development, and regeneration. Annu Rev Neurosci. 1991;14:301–333. - PubMed
-
- Lim DJ. Cochlear anatomy related to cochlear micromechanics: A review. J Acoust Soc Am. 1980;67(5):1686–1695. - PubMed
-
- Mann ZF, Kelley MW. Development of tonotopy in the auditory periphery. Hear Res. 2011;276(1-2):2–15. - PubMed
-
- Lee YS, Liu F, Segil N. A morphogenetic wave of p27Kip1 transcription directs cell cycle exit during organ of Corti development. Development. 2006;133(15):2817–2826. - PubMed
-
- Ruben RJ. Development of the inner ear of the mouse: A radioautographic study of terminal mitoses. Acta Otolaryngol. 1967;220 220, 1–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

