Protein phosphatase 2A enables expression of interleukin 17 (IL-17) through chromatin remodeling
- PMID: 23918926
- PMCID: PMC3772223
- DOI: 10.1074/jbc.M113.483743
Protein phosphatase 2A enables expression of interleukin 17 (IL-17) through chromatin remodeling
Abstract
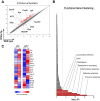
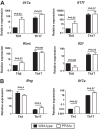
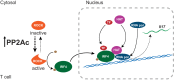
Protein phosphatase 2A (PP2A) is a heterotrimeric serine/threonine phosphatase involved in essential cellular functions. T cells from patients with systemic lupus erythematosus (SLE) express high levels of the catalytic subunit of PP2A (PP2Ac). A mouse overexpressing PP2Ac in T cells develops glomerulonephritis in an IL-17-dependent manner. Here, using microarray analyses, we demonstrate that increased expression of PP2Ac grants T cells the capacity to produce an array of proinflammatory effector molecules. Because IL-17 is important in the expression of glomerulonephritis, we studied the mechanism through which PP2Ac dysregulation facilitates its production. We report that PP2Ac is involved in the regulation of the Il17 locus by enhancing histone 3 acetylation through a mechanism that involves activation of interferon regulatory factor 4. Increased histone 3 acetylation of the Il17 locus is shared between T cells of PP2Ac transgenic mice and patients with SLE. We propose that, by promoting the inflammatory capacity of T cells, PP2Ac dysregulation contributes to the pathogenesis of SLE.
Keywords: Autoimmunity; Chromatin Histone Modification; Inflammation; Interleukin; PP2A; T Cell.
Figures




References
-
- Xu Y., Xing Y., Chen Y., Chao Y., Lin Z., Fan E., Yu J. W., Strack S., Jeffrey P. D., Shi Y. (2006) Structure of the protein phosphatase 2A holoenzyme. Cell 127, 1239–1251 - PubMed
-
- Cho U. S., Xu W. (2007) Crystal structure of a protein phosphatase 2A heterotrimeric holoenzyme. Nature 445, 53–57 - PubMed
-
- Janssens V., Longin S., Goris J. (2008) PP2A holoenzyme assembly. In cauda venenum (the sting is in the tail). Trends Biochem. Sci. 33, 113–121 - PubMed
-
- Lee T. Y., Lai T. Y., Lin S. C., Wu C. W., Ni I. F., Yang Y. S., Hung L. Y., Law B. K., Chiang C. W. (2010) The B56γ3 regulatory subunit of protein phosphatase 2A (PP2A) regulates S phase-specific nuclear accumulation of PP2A and the G1 to S transition. J. Biol. Chem. 285, 21567–21580 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

