Cryo-electron tomography: the challenge of doing structural biology in situ
- PMID: 23918936
- PMCID: PMC3734081
- DOI: 10.1083/jcb.201304193
Cryo-electron tomography: the challenge of doing structural biology in situ
Abstract
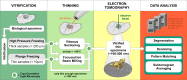
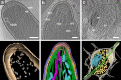
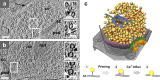
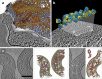
Electron microscopy played a key role in establishing cell biology as a discipline, by producing fundamental insights into cellular organization and ultrastructure. Many seminal discoveries were made possible by the development of new sample preparation methods and imaging modalities. Recent technical advances include sample vitrification that faithfully preserves molecular structures, three-dimensional imaging by electron tomography, and improved image-processing methods. These new techniques have enabled the extraction of high fidelity structural information and are beginning to reveal the macromolecular organization of unperturbed cellular environments.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

