Two phases of inflammatory mediator production defined by the study of IRAK2 and IRAK1 knock-in mice
- PMID: 23918981
- PMCID: PMC3849919
- DOI: 10.4049/jimmunol.1203268
Two phases of inflammatory mediator production defined by the study of IRAK2 and IRAK1 knock-in mice
Erratum in
- J Immunol. 2013 Nov 15;191(10):5317
Abstract
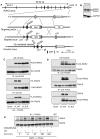
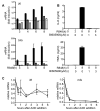
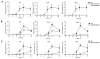
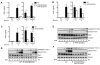
The roles of IL-1R-associated kinase (IRAK)2 and IRAK1 in cytokine production were investigated using immune cells from knock-in mice expressing the TNFR-associated factor 6 (TRAF6) binding-defective mutant IRAK2[E525A] or the catalytically inactive IRAK1[D359A] mutant. In bone marrow-derived macrophages (BMDMs), the IRAK2-TRAF6 interaction was required for the late (2-8 h) but not the early phase (0-2 h) of il6 and tnfa mRNA production, and hence for IL-6 and TNF-α secretion by TLR agonists that signal via MyD88. Loss of the IRAK2-TRAF6 interaction had little effect on the MyD88-dependent production of anti-inflammatory molecules produced during the early phase, such as Dual Specificity Phosphatase 1, and a modest effect on IL-10 secretion. The LPS/TLR4-stimulated production of il6 and tnfa mRNA and IL-6 and TNF-α secretion was hardly affected, because the Toll/IL-1R domain-containing adapter-inducing IFN-β (TRIF) signaling pathway was used instead of the IRAK2-TRAF6 interaction to sustain late-phase mRNA production. IRAK1 catalytic activity was not rate limiting for il6, tnfa, or il10 mRNA production or the secretion of these cytokines by BMDMs, but IFN-β mRNA induction by TLR7 and TLR9 agonists was greatly delayed in plasmacytoid dendritic cells (pDCs) from IRAK1[D359A] mice. In contrast, IFN-β mRNA production was little affected in pDCs from IRAK2[E525A] mice, but subsequent IFN-α mRNA production and IFN-α secretion were reduced. IFN-β and IFN-α production were abolished in pDCs from IRAK1[D359A] × IRAK2[E525A] double knock-in mice. Our results establish that the IRAK2-TRAF6 interaction is rate limiting for the late, but not the early phase of cytokine production in BMDM and pDCs, and that the IRAK2-TRAF6 interaction is needed to sustain IκB-inducing kinase β activity during prolonged activation of the MyD88 signaling network. [corrected]
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

