Epigenetic contributions to hormonally-mediated sexual differentiation of the brain
- PMID: 23919286
- PMCID: PMC5330673
- DOI: 10.1111/jne.12072
Epigenetic contributions to hormonally-mediated sexual differentiation of the brain
Abstract
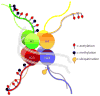
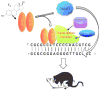
It has been long established that hormones exert enduring influences on the developing brain that direct the reproductive response in adulthood, although the cellular mechanisms by which organisational effects are maintained have not been determined satisfactorily. Recent interest in epigenetic modifications to the nervous system has highlighted the potential for hormone-induced changes to the genome that could endure for the lifespan but not be transmitted to the next generation. Preliminary evidence suggests that this is indeed possible because sex differences in the histone code and in the methylation of CpGs in the promoters of specific genes have been identified and, at times, functionally correlated with behaviour. The present review provides an overview of epigenetic processes and discusses the current state-of-the-art, and also identifies future directions.
Keywords: development; hypothalamus; oestrogens; preoptic area; sex differences; steroids: neuroactive steroids.
© 2013 British Society for Neuroendocrinology.
Figures

References
-
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. - PubMed
-
- Pandey R, Mukerji M. From ‘JUNK’ to just unexplored noncoding knowledge: the case of transcribed Alus. Brief Funct Genomics. 2011;10:294–311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

