A review of ceramide analogs as potential anticancer agents
- PMID: 23919551
- PMCID: PMC4216473
- DOI: 10.4155/fmc.13.107
A review of ceramide analogs as potential anticancer agents
Abstract
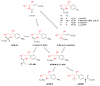
Ceramide serves as a central mediator in sphingolipid metabolism and signaling pathways, regulating many fundamental cellular responses. It is referred to as a 'tumor suppressor lipid', since it powerfully potentiates signaling events that drive apoptosis, cell cycle arrest, and autophagic responses. In the typical cancer cell, ceramide levels and signaling are usually suppressed by overexpression of ceramide-metabolizing enzymes or downregulation of ceramide-generating enzymes. However, chemotherapeutic drugs as well as radiotherapy increase intracellular ceramide levels, while exogenously treating cancer cells with short-chain ceramides leads to anticancer effects. All evidence currently points to the fact that the upregulation of ceramide levels is a promising anticancer strategy. In this review, we exhibit many anticancer ceramide analogs as downstream receptor agonists and ceramide-metabolizing enzyme inhibitors.
Figures


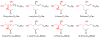


References
-
- Huwiler A, Zangemeister-Wittke U. Targeting the conversion of ceramide to sphingosine 1-phosphate as a novel strategy for cancer therapy. Crit Rev Oncol Hematol. 2007;63(2):150–159. - PubMed
-
- Huwiler A, Kolter T, Pfeilschifter J, Sandhoff K. Physiology and pathophysiology of sphingolipid metabolism and signaling. Biochim Biophys Acta. 2000;1485(2–3):63–99. - PubMed
-
- Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4(8):604–616. - PubMed
-
- Zhou H, Summers SA, Birnbaum MJ, Pittman RN. Inhibition of Akt kinase by cell-permeable ceramide and its implications for ceramide-induced apoptosis. J Biol Chem. 1998;273(26):16568–16575. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
