Calpain cleavage and inactivation of the sodium calcium exchanger-3 occur downstream of Aβ in Alzheimer's disease
- PMID: 23919677
- PMCID: PMC4326873
- DOI: 10.1111/acel.12148
Calpain cleavage and inactivation of the sodium calcium exchanger-3 occur downstream of Aβ in Alzheimer's disease
Abstract
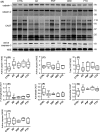
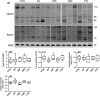
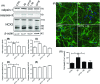
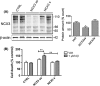
Alzheimer's disease (AD) is a neurodegenerative disorder characterized by pathological deposits of β-amyloid (Aβ) in senile plaques, intracellular neurofibrillary tangles (NFTs) comprising hyperphosphorylated aggregated tau, synaptic dysfunction and neuronal death. Substantial evidence indicates that disrupted neuronal calcium homeostasis is an early event in AD that could mediate synaptic dysfunction and neuronal toxicity. Sodium calcium exchangers (NCXs) play important roles in regulating intracellular calcium, and accumulating data suggests that reduced NCX function, following aberrant proteolytic cleavage of these exchangers, may contribute to neurodegeneration. Here, we show that elevated calpain, but not caspase-3, activity is a prominent feature of AD brain. In addition, we observe increased calpain-mediated cleavage of NCX3, but not a related family member NCX1, in AD brain relative to unaffected tissue and that from other neurodegenerative conditions. Moreover, the extent of NCX3 proteolysis correlated significantly with amounts of Aβ1-42. We also show that exposure of primary cortical neurons to oligomeric Aβ1-42 results in calpain-dependent cleavage of NCX3, and we demonstrate that loss of NCX3 function is associated with Aβ toxicity. Our findings suggest that Aβ mediates calpain cleavage of NCX3 in AD brain and therefore that reduced NCX3 activity could contribute to the sustained increases in intraneuronal calcium concentrations that are associated with synaptic and neuronal dysfunction in AD.
Keywords: Alzheimer's disease; beta-amyloid; calcium; calpain; sodium calcium exchanger; tau.
© 2013 the Anatomical Society and John Wiley & Sons Ltd.
Figures


References
-
- Ayala-Grosso C, Tam J, Roy S, Xanthoudakis S, Da Costa D, Nicholson DW, Robertson GS. Caspase-3 cleaved spectrin colocalizes with neurofilament- immunoreactive neurons in Alzheimer’s disease. Neuroscience. 2006;141:863–874. - PubMed
-
- Bano D, Young KW, Guerin CJ, Lefeuvre R, Rothwell NJ, Naldini L, Rizzuto R, Carafoli E, Nicotera P. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell. 2005;120:275–285. - PubMed
-
- Bano D, Munarriz E, Chen HL, Ziviani E, Lippi G, Young KW, Nicotera P. The plasma membrane Na+/Ca2+ exchanger is cleaved by distinct protease families in neuronal cell death. Ann. N. Y. Acad. Sci. 2007;1099:451–455. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

