Adverse drug reactions and off-label and unlicensed medicines in children: a prospective cohort study of unplanned admissions to a paediatric hospital
- PMID: 23919928
- PMCID: PMC4371534
- DOI: 10.1111/bcp.12222
Adverse drug reactions and off-label and unlicensed medicines in children: a prospective cohort study of unplanned admissions to a paediatric hospital
Abstract
Aims: To examine the impact of off-label and unlicensed (OLUL) prescribing on adverse drug reactions (ADRs) causing unplanned admissions to a paediatric hospital.
Methods: Prescription data from a 12 month prospective cohort study of ADRs detected in children admitted to a paediatric hospital were scrutinized. The relative risk for off-label and unlicensed medicines being implicated in an ADR was calculated. Logistic regression analyses were carried out with exposure to off-label and unlicensed medicines and number of off-label and unlicensed medicines administered as predictor variables.
Results: Off-label and unlicensed medicines were more likely to be implicated in an ADR than authorized medicines (relative risk 1.67, 95% CI 1.38, 2.02, P < 0.001). There was a 25% increase in ADR risk (95% CI 1.16, 1.35, P < 0.001) with each additional authorized medicine and 23% (95% CI 1.10, 1.36, P < 0.001) with each additional off-label or unlicensed medicine. Logistic regression analysis focusing on non-oncology patients demonstrated that the number of authorized medicines (odds ratio 1.33, 95% CI 1.23, 1.44, P < 0.001), but not the number of off-label and unlicensed medicine courses, was a predictor of ADR risk.
Conclusions: In a heterogeneous population of children admitted to a secondary/tertiary hospital, off-label and unlicensed medicines are more likely to be implicated in an ADR than authorized medicines. This was largely driven by ADRs related to drugs used in oncological practice, where the usage of off-label or unlicensed medicines was associated with a higher ADR risk than in non-oncological areas.
Keywords: ADR; off-label; paediatric; unlicensed.
© 2013 The British Pharmacological Society.
Figures


 , exposed to at least one OLUL medicine;
, exposed to at least one OLUL medicine;  , not exposed to OLUL medicine. ICH Classification of children by age (21): Preterm neonate <36 weeks gestation, 0–27 days (gestational age not recorded in our study); Neonate 0–27 days; Infants and toddlers 28 days–23 months; Children 2–11 years; Adolescents 12–17 years
, not exposed to OLUL medicine. ICH Classification of children by age (21): Preterm neonate <36 weeks gestation, 0–27 days (gestational age not recorded in our study); Neonate 0–27 days; Infants and toddlers 28 days–23 months; Children 2–11 years; Adolescents 12–17 years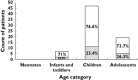
 , exposed to at least one OLUL medicine;
, exposed to at least one OLUL medicine;  , not exposed to OLUL medicine. ICH Classification of children by age (21): Preterm neonate <36 weeks gestation, 0–27 days (gestational age not recorded in our study); Neonate 0–27 days; Infants and toddlers 28 days–23 months; Children 2–11 years; Adolescents 12–17 years
, not exposed to OLUL medicine. ICH Classification of children by age (21): Preterm neonate <36 weeks gestation, 0–27 days (gestational age not recorded in our study); Neonate 0–27 days; Infants and toddlers 28 days–23 months; Children 2–11 years; Adolescents 12–17 yearsReferences
-
- Cuzzolin L, Atzei A, Fanos V. Off-label and unlicensed prescribing for newborns and children in different settings: a review of the literature and a consideration about drug safety. Expert Opin Drug Saf. 2006;5:703–718. - PubMed
-
- Impicciatore P, Mohn A, Chiarelli F, Pandolfini C, Bonati M. Adverse drug reactions to off-label drugs on a paediatric ward: an Italian prospective pilot study. Paediatr Perinat Drug Ther. 2002;5:19–24.
-
- Neubert A, Dormann H, Weiss J, Egger T, Criegee-Rieck M, Rascher W, Brune K, Hinz B. The impact of unlicensed and off-label drug use on adverse drug reactions in paediatric patients. Drug Safety. 2004;27:1059–1067. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

